Almost all patients said it was easy to add AUSTEDO XR to their daily routine
- More than half of patients (~55%) reported taking AUSTEDO XR in the morning
Almost all patients said it was easy to add AUSTEDO XR to their daily routine
Most patients (~84%) like the ability to adjust their dose up or down with their doctor
Patients in the pivotal and long-term studies received the AUSTEDO BID formulation.1,2
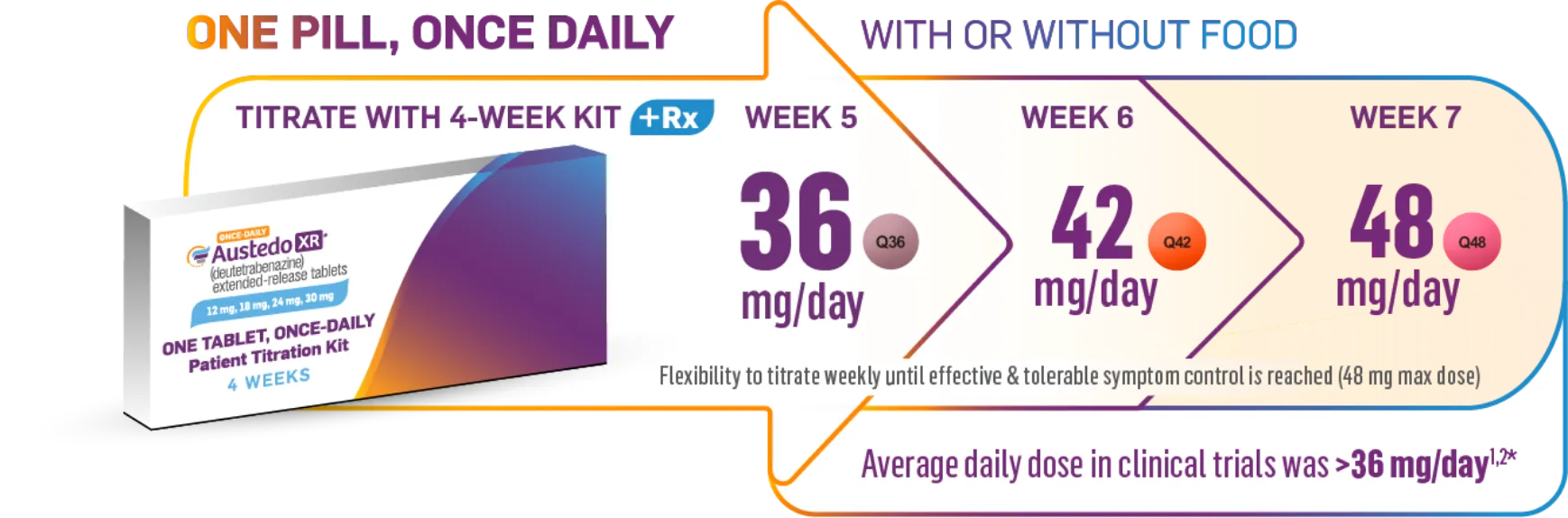
Image shown is not actual 4-week Titration Kit. Tablets not shown at actual size.
For more videos, visit the YouTube page.
[title]
EASING THE BURDEN FOR PATIENTS AND CAREGIVERS
NATHAN & HEATHER’S STORY
[description]
Easing the Burden for Patients and Caregivers
Nathan & Heather’s Story
Onscreen text:
EASING THE BURDEN FOR PATIENTS AND CAREGIVERS
NATHAN & HEATHER’S STORY
Disclaimer on bottom of screen:
This was an individual patient’s experience. Results may vary. Participants were compensated by Teva Pharmaceuticals.
[Blind footnote on bottom of screen]
HDc, Huntington’s disease chorea.
Heather:
I’m Heather. This is my husband, Nathan.
Nathan and I met through his sister, one of my best friends. We just adore each other. I don’t know any other way to put it.
Nathan:
I love her.
Heather:
He loves me.
Onscreen text:
Nathan and Heather were newlyweds when he was diagnosed with HD.
His chorea forced them to reconsider what their future together would look like.
Heather:
I knew nothing about Huntington’s disease until I met Nathan and his sister, and they would talk about their mom having the disease—it’s very scary.
Onscreen text:
Over time, Nathan’s symptoms worsened, and Heather soon became more than his wife—she became his caregiver.
Heather:
When Nathan first started needing my help, it was a bit more daunting. Our child was small, so I had them both depending on me.
He depended on me a lot…helping him walk, holding his hands. And I don’t mean the side by side holding hands, I mean I’m standing in front of him holding on to his arms to help him steady his gait.
Onscreen text:
In 2017, Nathan’s doctor started him on AUSTEDO.
Onscreen Indication and Black Box Warning:
AUSTEDO XR® and AUSTEDO® are indicated in adults for the treatment of chorea associated with Huntington's disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Please see additional Important Safety Information at the end of this video.
Heather:
We started seeing a difference once Nathan started AUSTEDO—he was able to hold on to tools, pick up drink cans, play with our kids.
Chorea control gives me peace of mind for how I care for Nathan.
I don’t feel quite as much as a caregiver. I feel more like a wife and more a partner with my husband.
Onscreen text:
When once-daily AUSTEDO XR became available, Nathan’s doctor recommended the switch.
Heather:
The process of switching to AUSTEDO XR was so easy.
It was very easy to explain the dose schedule to friends and family—you only have to take AUSTEDO XR once a day, with or without food. It made life a whole lot easier.
We didn’t have to change any doses with his other medications.
We love that we can increase and decrease the dosages as needed.
Onscreen text:
Despite his HD progressing, Nathan continues to see chorea control more than 8 years after starting treatment.
Heather:
With disease progression, movement control gives us a better bond because we can sit and hold hands, we can enjoy watching a movie—we can enjoy things that we felt like we would never be able to enjoy again.
He can play video games and bond with our son.
We attribute AUSTEDO XR to helping him out considerably. I have my husband back a little bit again.
Onscreen text:
DISCOVER THE DIFFERENCE CHOREA MANAGEMENT CAN MAKE FOR PATIENTS AND THEIR CAREGIVERS AT AUSTEDOhcp.com
Onscreen ISI scroll
INDICATIONS AND USAGE
AUSTEDO XR and AUSTEDO are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, at AUSTEDOhcp.com.

Ask your Sales Rep for samples. Notify
pharmacy if patient has been sampled.
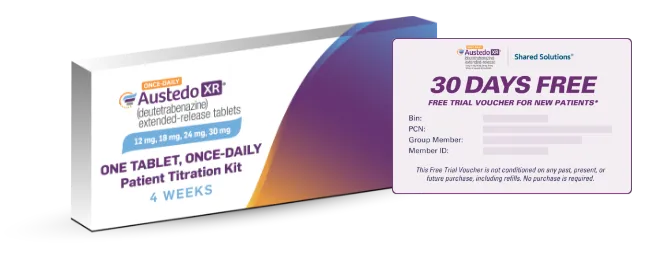
Pharmacy will dispense prescribed kit
and apply 30-day Free Trial Voucher.
Titration Kit NDC: 68546-0477-29
Continue titrating weekly until symptom control is effectively and tolerably achieved (48 mg/day maximum dosage).1
*Certain restrictions apply. Terms and conditions on AUSTEDOcardform.com.
Treatment success consistent with pivotal studies (PGIC/CGIC)†
Easy-to-follow titration schedule
Overall satisfaction with kit
CGIC, Clinical Global Impression of Change; PGIC, Patient Global Impression of Change.
†Treatment success defined as “much improved” or “very much improved” based on PGIC and CGIC.4
‡The START study was a non-interventional, real-world study assessing 4-week Titration Kit utilization and treatment success in 53 patients with tardive dyskinesia and 17 patients with Huntington’s disease chorea, both of similar demographics to the AUSTEDO pivotal studies.4,5
A guide with access and affordability information to
get patients started on AUSTEDO XR.
Download the Appeals Letter Template
Download the Letter of Medical Necessity Template
REFERENCES: 1. AUSTEDO XR® (deutetrabenazine) extended-release tablets and AUSTEDO® current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc. 2. Frank S, Testa C, Edmondson MC, et al. The safety of deutetrabenazine for chorea in Huntington disease: an open-label extension study. CNS Drugs. 2022;36(11):1207-1216. 3. Bulica B, Konings M, Thompson S, Yang A, Kotak S, Gandhi P. Patient experience and satisfaction with once-daily deutetrabenazine extended-release tablets for treatment of Huntington disease-related chorea. Poster presented at: Annual Contemporary Neurology Congress; January 18-21, 2025; Clearwater, FL. 4. Data on file. Parsippany, NJ: Teva Neuroscience, Inc. 5. Anderson KE, Konings M, Finkbeiner S, et al. Treatment patterns, effectiveness, and satisfaction with deutetrabenazine in Huntington disease when initiated using a 4-week patient titration kit: interim results of the START Study. Poster presented at: Huntington Study Group® (HSG) Annual Meeting; November 2-4, 2023; Phoenix, AZ.