Resources for each step of the treatment journey with
AUSTEDO XR
Downloadable resources
AUSTEDO XR
Fill out this form to enroll your patient in Teva Shared Solutions® (for patients taking AUSTEDO XR).
Fill out this form to enroll your patient in Shared Solutions (for patients taking AUSTEDO BID).
Use this template to write an appeals letter, which payers may need in order to appeal a denial of coverage for AUSTEDO XR or AUSTEDO tablets.
Use this template to write a letter to establish medical necessity, which payers may require for treatment with AUSTEDO XR or AUSTEDO.
This brochure provides an overview of Shared Solutions support for your patients and outlines how to enroll.
Use this manual when prescribing AUSTEDO XR in the PointClickCare platform.
A guide with access and affordability information to get patients started on AUSTEDO XR.
This flashcard outlines improvements to Medicare made by the Inflation Reduction Act (IRA) to expand benefits and improve drug costs for many patients.
Downloadable resources for your patients with HD chorea
This brochure provides answers to important questions your patients with HD chorea and their care partners may have when starting treatment.
A useful resource for patients as they begin their treatment journey. Patients and their care partners can use this tracking tool to record when they take their treatment, as well as any changes in their HD chorea.
This brochure provides information about financial assistance options available through Shared Solutions, including the 30-day Free Trial Voucher and $0 copay card.
This resource is for patients to use at their next appointment with you—whether in person or through telemedicine—to help decide if AUSTEDO XR is right for them.
A resource introducing patients to Shared Solutions—a patient support partner that can help when getting started with AUSTEDO XR, and with finding financial assistance offerings to stay on treatment.
Videos to help you support your patients with HD chorea
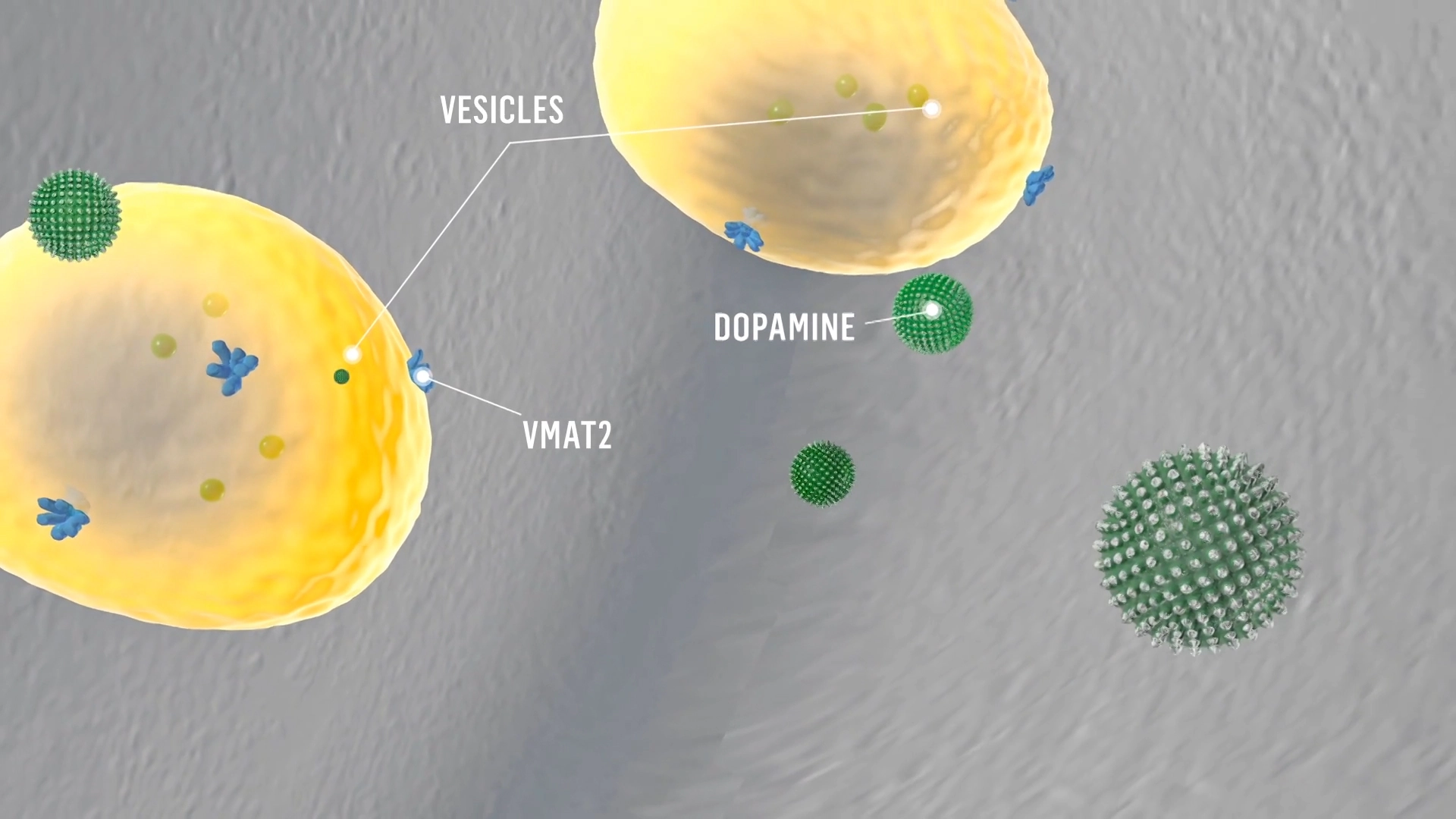
Mechanism of Action
VMAT2 inhibition can help regulate dopamine function.1
Voiceover:
Maintaining a balance in the level of dopamine is essential for controlled movement.
Dopamine signaling is facilitated by vesicular monoamine transporter 2, or VMAT2, which transports monoamines, including dopamine, from the cytosol into synaptic vesicles, keeping them ready for subsequent release in response to an action potential.
When an action potential reaches the nerve terminal of the presynaptic neuron, dopamine is released from the synaptic vesicles into the synaptic cleft.
This dopamine binds to receptors on the postsynaptic neuron, thereby signaling movement.
Excess dopamine signaling manifests as abnormal involuntary movements.
AUSTEDO® (deutetrabenazine) is a VMAT2 inhibitor.
Deutetrabenazine contains deuterium, a naturally occurring heavy version of hydrogen. Deuterium forms stronger molecular bonds with carbon atoms, thereby extending the half-life of therapeutic metabolites.
The precise mechanism by which deutetrabenazine exerts its effects on abnormal involuntary movements is unknown. It is believed to be related to its effect as a reversible depleter of monoamines, including dopamine, from nerve terminals.
Deutetrabenazine binds to VMAT2 on the vesicle in the presynaptic neuron and inhibits the uptake of dopamine into synaptic vesicles.
Dopamine molecules collect outside the blocked VMAT2 and are degraded by monoamine oxidase.
Reducing dopamine levels in the presynaptic neuron results in less dopamine signaling to the postsynaptic neuron.
Limiting dopamine signaling is believed to lead to fewer abnormal involuntary movements.

The Impact of HD Chorea
Eileen discusses how her life, and in particular, her work, has been affected by HD chorea.
Onscreen text:
A patient talks about how she had to stop work as a legal secretary due to her Huntington’s disease.
Eileen:
Well, it affected me and my working.
I’m not working anymore, I’m on disability, and I, uh…
It’s…’cause I can’t read as much, like I used to read. Um…
Rajeev Kumar:
What kind of work did you used to do?
Eileen:
I was a legal secretary.
Rajeev Kumar:
And how did it interfere with reading and doing your job?
Eileen:
It interfered with my reading pleadings and emails, um.
I can’t comprehend reading anymore. And I’ve been reading my whole life.
Um, I don’t read books like I used to.
In fact, I don’t read them at all.
And it has affected me in ways of eating.
I drop my food, I drop…I fall over. I fall on occasion.

Hear From an Expert: Pivotal Trial Results
Dr. Rajeev Kumar discusses SF-36 results and impact on activities of daily living.
Voiceover/Onscreen text:
INDICATIONS AND USAGE
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease:
AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Please see additional Important Safety Information at the end of this video.
Rajeev Kumar:
Hello. I’m Dr. Rajeev Kumar, Medical Director of the Rocky Mountain Movement Disorders Center and Huntington’s Disease Society of America Center of Excellence in Colorado. For over 20 years, I have been working with patients to treat their chorea symptoms.
I was involved in the evaluation of AUSTEDO in the pivotal trial for treatment of chorea associated with HD. I chose to be involved because the success of this trial could lead to a treatment for my patients with chorea.
I had a conversation with a colleague the other day about our patients with HD chorea. AUSTEDO is an appropriate choice to treat those symptoms, and I thought it’d be insightful to share some of our discussion with you.
My colleague said he was concerned about the impact chorea symptoms were having on his patients and their caregivers. He was particularly concerned about patients who were not aware of their symptoms and had become increasingly reliant on their caregivers. Even when patients are not aware of their symptoms, it is still important to treat them because of the impact chorea can have on their ability to function and their need for daily assistance.
The efficacy and safety of AUSTEDO were demonstrated in a randomized, 12-week placebo-controlled study in patients with chorea. The primary endpoint was total maximal chorea, or TMC, score. AUSTEDO reduced TMC score by 4.4 points from baseline, more than twice the improvement in the TMC score from placebo. The reduction in chorea was demonstrated in the face, mouth, trunk, and legs.
In my experience, after patients begin treatment, they often acknowledge improvements in areas of self-care and activities of daily living. My colleague and I talked about how we have observed improvement in our patients first-hand—particularly in areas of daily living, including walking, bathing, dressing, lifting, carrying groceries, moderate to vigorous activities, or climbing stairs.
Both patients and clinicians reported treatment success as measured by the Patient Global Impression of Change, or PGIC, and Clinical Global Impression of Change, or CGIC. Treatment is considered successful when symptoms are rated as “much improved” or “very much improved.” On the PGIC, the patient success rate with AUSTEDO was 51 percent, compared with 20 percent for placebo.
The physical function portion of the Short-Form 36 Health Survey, known as the SF-36, supported these findings as well. In the clinical trial for AUSTEDO, there was a statistically significant reduction in disability and improvement in physical functioning compared with placebo at 12 weeks.
While we were talking about study results, I mentioned that I tell my patients the most common side effects can include drowsiness, dry mouth, diarrhea, and fatigue. And I encourage them to share with me any symptoms they find bothersome.
Disclaimer at bottom of screen:
Patients who participated in the clinical trials discussed in this testimonial received the BID formulation of AUSTEDO.
Rajeev Kumar:
In my practice, I have been pleased with the experience my patients with HD chorea have had with AUSTEDO, particularly with their improvements in areas of daily living, physical functioning, and reduced disability. In addition to managing symptoms, I am always looking for opportunities to offer my patients a choice. With the availability of AUSTEDO XR, my patients have the option to take their treatment once daily.
Voiceover/Onscreen text:
INDICATIONS AND USAGE
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease:
AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics, The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO. Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, at AUSTEDOhcp.com.

Hear From an Expert: ARC-HD Trial Results
Dr. Fahd Amjad discusses long-term results through 3 years.
Voiceover/Onscreen text:
INDICATIONS AND USAGE
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Please see additional Important Safety Information at the end of this video.
Fahd Amjad:
Hello. I am Dr. Fahd Amjad, Co-Director of the Huntington’s Disease Care, Education, Research Center at Georgetown University Medical Center in Washington, DC.
For over 10 years, I have led our mission to provide treatment to and manage the symptoms experienced by patients with Huntington’s disease, or HD.
I ran into a colleague the other day, and thought I would share the conversation we had about our patients with HD chorea. More than 90 percent of patients with HD have chorea, and as neurologists, we appreciate the challenges faced by patients with chorea symptoms.
We both regularly observe that patients with chorea have difficulty walking and may fall. My colleague recently started managing a new patient with chorea symptoms and wanted to understand what my goals are for patients. Because patients with chorea are at risk of falls, they often become dependent on their partners or caregivers for help with their daily activities. One of my goals is to help patients maintain their independence for as long as possible.
I asked my colleague what he prescribes for his patients with chorea. Both of us consider AUSTEDO, a vesicular monoamine transporter 2 inhibitor, or VMAT2, for the management of chorea symptoms in patients with HD.
I choose AUSTEDO because its efficacy and safety were demonstrated in a randomized, 12-week, placebo-controlled study in patients with chorea associated with HD. The results showed a statistically significant reduction in chorea of the face, mouth, trunk, arms, and legs as measured by the total maximal chorea score.
AUSTEDO is also the first VMAT2 inhibitor to have physicians and patients rate overall HD chorea symptoms as “much improved” or “very much improved” when compared to placebo as measured by the Clinical Global Impression of Change and Patient Global Impressions of Change scales.
I mentioned to him that I recently reviewed a 3-year open-label extension study called ARC-HD, published in CNS Drugs. This AUSTEDO study interests me as a provider because HD is a progressive condition, and the study demonstrated about 3 years of continued control of chorea in patients.
Disclaimer at bottom of page:
Patients who participated in the clinical trials discussed in this testimonial received the BID formulation for AUSTEDO.
Fahd Amjad:
I am also confident in the demonstrated safety profile of AUSTEDO and explain to my patients that the most common side effects include somnolence, diarrhea, dry mouth, and fatigue. These side effects occur most often during the dosing titration phase.
Most of my patients are on an average daily of 36 mg with some up to 48 mg, which aligns with the clinical trials for AUSTEDO. And I can also offer my patients more options with the introduction of AUSTEDO XR, a once-daily formulation.
Voiceover/Onscreen text:
INDICATIONS AND USAGE
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics, The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, at AUSTEDOhcp.com.

Moves That Matter: Ray & Rhonda’s Long-Term Journey With HD Chorea
Ray:
I’m Ray…I’m 55.
Rhonda:
My name is Rhonda, and I am Ray’s wife.
Disclaimer at bottom of screen:
This was an actual patient’s experience. Results may vary. Ray and Rhonda were compensated for their participation in this video.
Onscreen text:
Ray and Rhonda were looking forward to spending their retirement hiking, traveling, and enjoying their grandchildren.
But when Ray was diagnosed with HD in 2019, it seemed like chorea would make all of that impossible.
Rhonda:
Ray’s dad had Huntington’s disease…he wasn’t able to walk…he ended up in a wheelchair…
So that was kind of a really scary thought for me. I just assumed that our lives…would be over very soon as we knew it…
Onscreen text:
As Ray’s disease progressed, his chorea began to affect him day and night.
Rhonda:
So the first symptoms I noticed of his chorea was at night while he was sleeping. His arms would, you know, kind of start flailing and hitting and then kind of it would just be like his whole body…
Ray:
Yeah. When I was driving to work one day…and my hands are just shaking like this…
But yeah, it’s just weird, you know, having your hands move, no explanation…
Rhonda:
At that time, I was feeling a little bit desperate, like, something needs to change. We can’t do this anymore.
Onscreen text:
When Ray fell out of bed and dislocated his shoulder, Rhonda realized it was time to talk to Ray’s doctor.
Rhonda:
We made an appointment with the neurologist that week talking to her about how bad it had gotten.
And she said, well, I have this drug that I would like for you to try.
Onscreen text:
Ray’s doctor started him on AUSTEDO, and as his dose was titrated, Ray and Rhonda began noticing an improvement in Ray’s chorea.
More than 3 years later, Ray is still taking AUSTEDO at 48 mg/day. His chorea movements have remained minimal.
Disclaimer at bottom of screen:
Once-daily AUSTEDO XR is available.
Bioequivalence of once-daily AUSTEDO XR has been established with AUSTEDO BID based on pharmacokinetic profile studies.
When two formulations are shown to be bioequivalent, they are considered to be therapeutically equivalent.
Rhonda:
Yeah. So his movements just became less and less as…the doses increased…
His sleeping now is very different…no more kicks, no more punches, no more flailing around in bed.
And…we both noticed that the small things that he would do with his hands became easier.
Ray:
Yeah.
Rhonda:
Buttoning his shirt, putting the leash on the dog’s collar, tying shoes. Those things became easier.
So yeah…it has far exceeded our expectations…
Onscreen text:
Today, Ray and Rhonda are still on the move—enjoying an active lifestyle and making the most of each day together.
Rhonda:
We’re looking forward to taking a trip to Yellowstone, hopefully taking our grandkids and doing some hiking…
I don’t know that we would have been able to continue hiking with his chorea had it continued on without the medication…
I feel like that we’re actually like living the way we’ve always wanted to live now…
…by going on vacations and being outdoors a lot more and having grandkids…
And yeah, I can’t image life if he didn’t have the medication.
Ray:
It really is a deal-maker for me. It just helped so much.
Onscreen text:
WHAT COULD LONG-TERM RESULTS MEAN FOR YOUR PATIENTS WITH HD CHOREA?
Learn more at AUSTEDOhcp.com
Onscreen ISI scroll:
INDICATIONS AND USAGE
AUSTEDO XR and AUSTEDO are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease:
AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO. Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, on AUSTEDOhcp.com.
Onscreen text:
This was an individual patient’s experience. Results may vary.
Reference: Data on file. Parsippany, NJ: Teva Neuroscience, Inc.
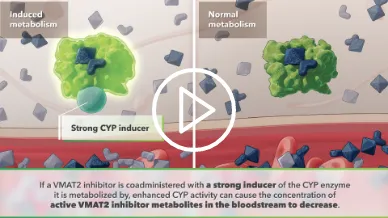
Metabolic Pathways
Visualizing the role of metabolic pathways in drug-drug interactions (DDIs)
Onscreen text:
CYP enzymes mediate the breakdown of active drug to inactive metabolites for clearance.
Together, CYP3A4/5 and CYP2D6 enzymes are involved in metabolizing ~75% drugs.
CYP enzymes play a key role in converting VMAT2 inhibitors into their active and inactive metabolites.
Certain drugs can act as strong CYP inhibitors or inducers, affecting the metabolism of coadministered medications…
…resulting in drug-drug interactions.
Inhibited metabolism
Inhibited metabolism results in elevated levels of active metabolites.
Elevated levels of active metabolites lead to increased drug potency and potential for adverse events.
Induced metabolism
Induced metabolism results in decreased levels of active metabolites.
Decreased levels of active metabolites diminish therapeutic effect.
For VMAT2 inhibitors, drug-drug interactions are determined by:
The CYP enzymes critical in metabolizing the VMAT2 inhibitor
AND
The metabolic profiles of the medications the patient is taking concomitantly (whether they are strong inhibitors or inducers)
To avoid drug-drug interactions associated with inhibited or induced metabolism, limiting VMAT2 inhibitor dose or avoiding use with strong inhibitors and inducers may be recommended.
When choosing a VMAT2 inhibitor, considering these drug-drug interactions is key.
VMAT2, vesicular monoamine transporter 2.
The Impact of HD Chorea on Daily Activities: Ray and Rhonda’s Story
Onscreen text:
HD chorea
THE IMPACT OF HD CHOREA ON DAILY ACTIVITIES
RAY & RHONDA’S STORY
Disclaimer at bottom of screen:
This was an individual patient’s experience. Results may vary. Ray and Rhonda were compensated for their participation in this video.
Onscreen text:
HD, Huntington’s disease.
Rhonda:
As the movements progressed more and more, I was thinking about what equipment he would need for getting dressed or taking a shower. OR if he would need a wheelchair.
On-screen text:
99% of patients with HD chorea require assistance with self-care activities.
When Ray was diagnosed with HD in 2019, his chorea had already begun limiting his physical functioning.
Ray:
I first noticed my involuntary movements when we were working…and my hand would just shake. I was just thinking, what’s going on?
Rhonda:
I was noticing at night when we were sleeping…he would be kicking me or hitting…he was just flailing around, moving his arms and legs.
Onscreen text:
As Ray’s symptoms worsening, Ray and his wife, Rhonda, knew it was time to talk to his doctor.
Rhonda:
We spoke with the doctor about it, and we didn’t know if there was any options for dealing with the chorea or not.
But we did talk to her and let her know that this has been going on for quite some time, and just wondering if there was any help for that.
Onscreen text:
The physical functioning portion of the SF-36 questionnaire, a health-related quality of life scale, was used to assess the impact of Ray’s chorea on daily activities.
Bathing
Lifting/carrying groceries
Walking
Moderate to vigorous activities
Climbing stairs
Bending, kneeling, or stooping
SF-36, Short Form Health Survey.
Based on Ray’s symptoms and the impact his chorea had on his activities of daily living, he was prescribed AUSTEDO.
Onscreen Indication and Black Box Warning:
AUSTEDO XR® and AUSTEDO® are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington's disease.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Please see additional Important Safety Information at the end of this video.
Onscreen text:
Patients taking AUSTEDO, like Ray, reported being better able to perform activities of daily living—with a significant 4.3-point difference in SF-36 physical functioning score in the pivotal trial.
Patients taking placebo had a decline in physical functioning as measured by SF-36
Rhonda:
He’s able to still do all the daily activities that one does during the day, all the ordinary things that sometimes the rest of us would take for granted.
Onscreen text:
More than 3 years later, Ray is still taking AUSTEDO at 48 mg/day. His chorea movements have remained minimal, and he’s better able to perform activities of daily living.
Disclaimer at bottom of screen:
Once-daily AUSTEDO XR is available.
Bioequivalence of once-daily AUSTEDO XR has been established with AUSTEDO BID based on pharmacokinetic profile studies. When two formulations are shown to be bioequivalence, they are considered to be therapeutically equivalent.
Rhonda:
Starting early on AUSTEDO has made a big difference in what we’re able to do now.
It keeps his movements at bay, keeps us hiking and walking and traveling and doing things that I don’t believe we would be able to do had he not got it started.
We’re actually like living the way we’ve always wanted to live now. I can’t imagine life if he didn’t have it.
Onscreen text:
Learn more about how AUSTEDO could make a different for your patients’ physical functioning at AUSTEDOhcp.com
Onscreen ISI scroll:
INDICATIONS AND USAGE
AUSTEDO XR and AUSTEDO are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington's disease.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity.
Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, at AUSTEDOhcp.com.
Onscreen text:
This was an individual patient’s experience. Results may vary.
References: 1. Claassen DO, DeCourcy J, Mellor J, Johnston C, Iyer RG. Impact of chorea on self-care activity, employment, and health-care resource use in patients with Huntington’s disease. J Health Econ Outcomes Res. 2021;8(1):99-105. 2. Data on file. Parsippany, NJ: Teva Neuroscience, Inc. 3. RAND Corporation. 36-Item Short Form Survey Instrument (SF-36). RAND Corporation. Accessed November 12, 2022. https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/survey-instrument.html

Easing the Burden for Patients and Caregivers: Nathan & Heather’s HD Chorea Journey
Onscreen text:
EASING THE BURDEN FOR PATIENTS AND CAREGIVERS
NATHAN & HEATHER’S STORY
Disclaimer on bottom of screen:
This was an individual patient’s experience. Results may vary. Participants were compensated by Teva Pharmaceuticals.
[Blind footnote on bottom of screen]
HDc, Huntington’s disease chorea.
Heather:
I’m Heather. This is my husband, Nathan.
Nathan and I met through his sister, one of my best friends. We just adore each other. I don’t know any other way to put it.
Nathan:
I love her.
Heather:
He loves me.
Onscreen text:
Nathan and Heather were newlyweds when he was diagnosed with HD.
His chorea forced them to reconsider what their future together would look like.
Heather:
I knew nothing about Huntington’s disease until I met Nathan and his sister, and they would talk about their mom having the disease—it’s very scary.
Onscreen text:
Over time, Nathan’s symptoms worsened, and Heather soon became more than his wife—she became his caregiver.
Heather:
When Nathan first started needing my help, it was a bit more daunting. Our child was small, so I had them both depending on me.
He depended on me a lot…helping him walk, holding his hands. And I don’t mean the side by side holding hands, I mean I’m standing in front of him holding on to his arms to help him steady his gait.
Onscreen text:
In 2017, Nathan’s doctor started him on AUSTEDO.
Onscreen Indication and Black Box Warning:
AUSTEDO XR® and AUSTEDO® are indicated in adults for the treatment of chorea associated with Huntington's disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Please see additional Important Safety Information at the end of this video.
Heather:
We started seeing a difference once Nathan started AUSTEDO—he was able to hold on to tools, pick up drink cans, play with our kids.
Chorea control gives me peace of mind for how I care for Nathan.
I don’t feel quite as much as a caregiver. I feel more like a wife and more a partner with my husband.
Onscreen text:
When once-daily AUSTEDO XR became available, Nathan’s doctor recommended the switch.
Heather:
The process of switching to AUSTEDO XR was so easy.
It was very easy to explain the dose schedule to friends and family—you only have to take AUSTEDO XR once a day, with or without food. It made life a whole lot easier.
We didn’t have to change any doses with his other medications.
We love that we can increase and decrease the dosages as needed.
Onscreen text:
Despite his HD progressing, Nathan continues to see chorea control more than 8 years after starting treatment.
Heather:
With disease progression, movement control gives us a better bond because we can sit and hold hands, we can enjoy watching a movie—we can enjoy things that we felt like we would never be able to enjoy again.
He can play video games and bond with our son.
We attribute AUSTEDO XR to helping him out considerably. I have my husband back a little bit again.
Onscreen text:
DISCOVER THE DIFFERENCE CHOREA MANAGEMENT CAN MAKE FOR PATIENTS AND THEIR CAREGIVERS AT AUSTEDOhcp.com
Onscreen ISI scroll
INDICATIONS AND USAGE
AUSTEDO XR and AUSTEDO are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, at AUSTEDOhcp.com.
For more videos, visit the YouTube page.
SF-36, Short Form (36) Health Survey; VMAT2, vesicular monoamine transporter 2.
PointClickCare® is a registered trademark of PointClickCare Technologies Inc.
REFERENCE: 1. Solmi M, Pigato G, Kane JM, Correll CU. Treatment of tardive dyskinesia with VMAT-2 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:1215-1238.