Resources for each step of the treatment journey with AUSTEDO XR
Downloadable resources
Use this tool for the assessment and routine monitoring of patients at risk of TD.
A tool developed and published by a consensus panel of experts to help guide the routine monitoring and evaluation of TD impact.
A tool to help HCPs and office staff assess and identify patients’ involuntary movements.
AUSTEDO XR
Fill out this form to enroll your patient in Teva Shared Solutions® (for patients taking AUSTEDO XR).
Fill out this form to enroll your patient in Shared Solutions (for patients taking AUSTEDO BID).
Use this template to write an appeals letter, which payers may need in order to appeal a denial of coverage for AUSTEDO XR or AUSTEDO tablets.
Use this template to write a letter to establish medical necessity, which payers may require for treatment with AUSTEDO XR or AUSTEDO.
This brochure provides an overview of Shared Solutions support for your patients and outlines how to enroll.
A guide with access and affordability information to get patients started on AUSTEDO XR.
This flashcard outlines improvements to Medicare made by the Inflation Reduction Act (IRA) to expand benefits and improve drug costs for many patients.
Downloadable resources for your patients with TD
This brochure provides answers to important questions your patients with TD and their care partners may have when starting treatment.
A useful resource for patients as they begin their treatment journey. Patients and their care partners can use this tracking tool to record when they take their treatment, as well as any changes in their TD.
This brochure provides information about financial assistance options available through Shared Solutions, including the 30-day Free Trial Voucher and $0 copay card.
This resource is for patients to use at their next appointment with you—whether in person or through telemedicine—to help decide if AUSTEDO XR is right for them.
A resource introducing patients to Shared Solutions—a patient support partner that can help when getting started with AUSTEDO XR, and with finding financial assistance offerings to stay on treatment.
Videos to help you support your patients with TD
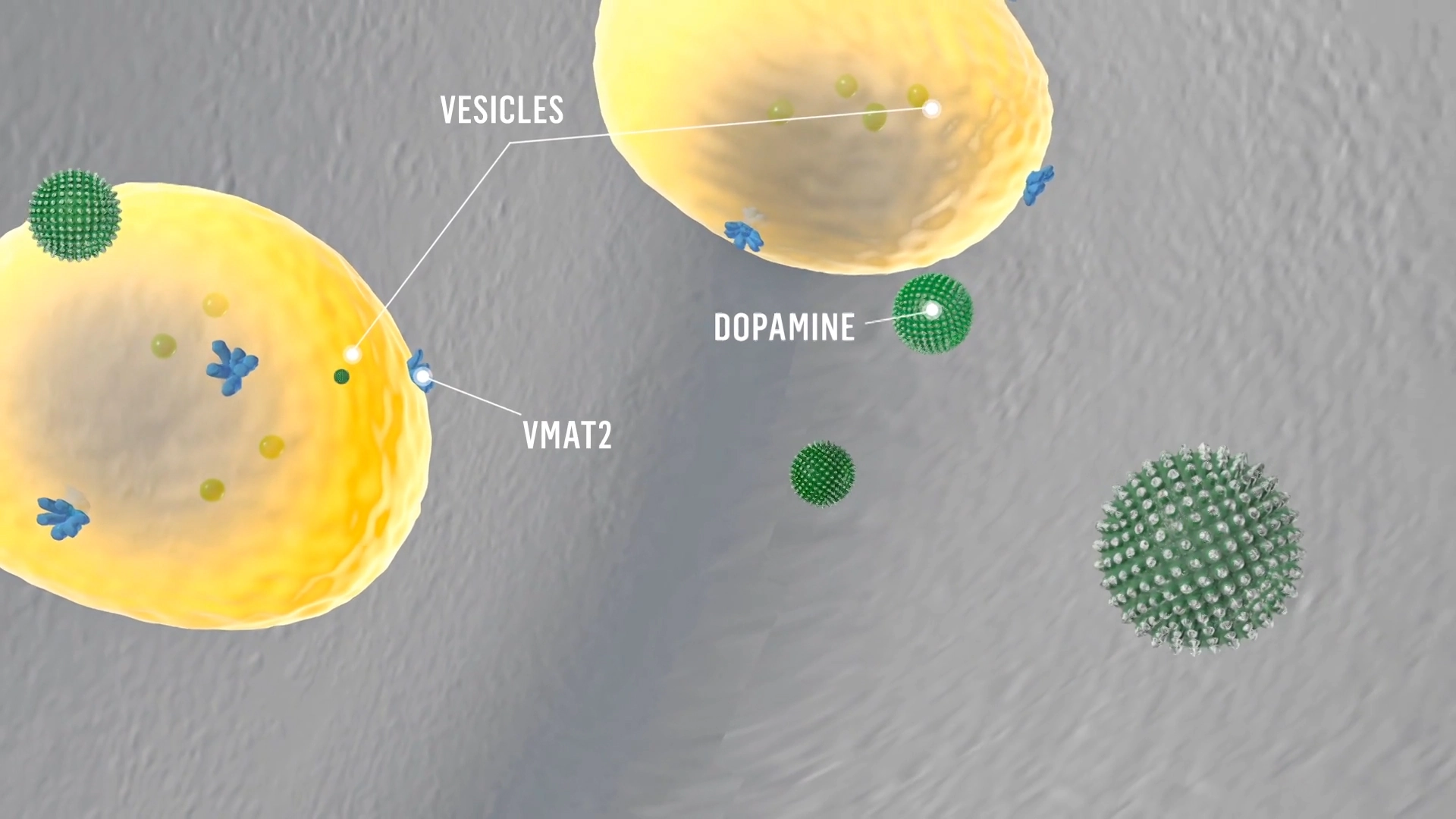
Mechanism of Action
VMAT2 inhibition can help regulate dopamine function.1
Voiceover:
Maintaining a balance in the level of dopamine is essential for controlled movement.
Dopamine signaling is facilitated by vesicular monoamine transporter 2, or VMAT2, which transports monoamines, including dopamine, from the cytosol into synaptic vesicles, keeping them ready for subsequent release in response to an action potential.
When an action potential reaches the nerve terminal of the presynaptic neuron, dopamine is released from the synaptic vesicles into the synaptic cleft.
This dopamine binds to receptors on the postsynaptic neuron, thereby signaling movement.
Excess dopamine signaling manifests as abnormal involuntary movements.
AUSTEDO® (deutetrabenazine) is a VMAT2 inhibitor.
Deutetrabenazine contains deuterium, a naturally occurring heavy version of hydrogen. Deuterium forms stronger molecular bonds with carbon atoms, thereby extending the half-life of therapeutic metabolites.
The precise mechanism by which deutetrabenazine exerts its effects on abnormal involuntary movements is unknown. It is believed to be related to its effect as a reversible depleter of monoamines, including dopamine, from nerve terminals.
Deutetrabenazine binds to VMAT2 on the vesicle in the presynaptic neuron and inhibits the uptake of dopamine into synaptic vesicles.
Dopamine molecules collect outside the blocked VMAT2 and are degraded by monoamine oxidase.
Reducing dopamine levels in the presynaptic neuron results in less dopamine signaling to the postsynaptic neuron.
Limiting dopamine signaling is believed to lead to fewer abnormal involuntary movements.

Improvement Over Time
See an update on Charlene and her treatment journey 2+ years later.
Onscreen text/quote bubbles:
Improvement Over Time: An Update on Charlene 2+ Years Later
Age: 65
Primary condition: Schizoaffective disorder
Disclaimer at bottom of screen:
This was an individual patient’s experience on AUSTEDO BID. Results may vary.
Onscreen text/quote bubbles:
“After a little over two years on AUSTEDO…my feet aren’t moving all the time. My hands aren’t moving all the time.”
“I can do something else with my hands now. I can tie my shoes…I can do things that people consider normal, that weren’t normal for me.”
CHARLENE’S JOURNEY OVER 2+ YEARS
When Charlene developed TD, she was told her involuntary movements would last forever. But she wasn’t ready to give up…
After starting AUSTEDO, Charlene began to see an improvement in her involuntary movements.
“I used to move my fingers without realizing, and I rocked…and I’m not doing that hardly at all…it is better.” – Charlene
“So do you think we’re at the right dose…or do you think we still have room for improvement?” – HCP
“I think there’s room for improvement.” – Charlene
As her dose was increased, Charlene’s movements continued to improve.
“Your movements look better…your fingers look pretty stable…” – HCP
“Yeah, and my mouth isn’t going 20 miles per hour…” – Charlene
Charlene’s doctor increased her dose to 48 mg/day, where Charlene experienced effective and tolerable symptom improvement.
Now, more than 2 years after starting treatment, Charlene can’t imagine going back to life without AUSTEDO.
“After a little over two years on AUSTEDO…my feet aren’t moving all the time. My hands aren’t moving all the time.” – Charlene
“I can do something else with my hands now. I can tie my shoes…I can do things that people consider normal, that weren’t normal for me.” – Charlene
“I don’t want to go back to the motions, so I prefer to stay on AUSTEDO…it helps and I don’t want to lose that.” – Charlene
Disclaimer at bottom of screen:
No clinical trials have been conducted to suggest that AUSTEDO affects the outcomes listed above.
Onscreen text/quote bubbles:
What could long-term results mean for your patients like Charlene?
See the rest of Charlene’s story at AUSTEDOhcp.com
Disclaimer at bottom of screen:
Once-daily AUSTEDO XR Is available.
Bioequivalence of once-daily AUSTEDO XR has been established with AUSTEDO BID based on pharmacokinetic profile studies. When two formulations are shown to be bioequivalent, they are considered to be therapeutically equivalent.
Onscreen ISI scroll:
INDICATIONS AND USAGE
AUSTEDO XR (deutetrabenazine) extended-release tablets and AUSTEDO (deutetrabenazine) tablets are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets. Please see full Prescribing Information, including Boxed Warning, on AUSTEDOhcp.com.
Onscreen text: This was an individual patient’s experience. Results may vary.
REFERENCES: AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc. Chow SC. Bioavailability and bioequivalence in drug development. Wiley Interdiscip Rev Comput Stat. 2014;6(4):304-312. Data on file. Parsippany, NJ: Teva Neuroscience, Inc.

The Impact of TD: Charlene and Squirt
When Charlene’s TD was at its worst, the movements in her hands, legs, and feet got in the way of her caring for her best friend, Squirt. See why AUSTEDO was the right move for her.
Charlene:
My dog, he’s a Chihuahua terrier.
Onscreen text:
The Impact of TD: Charlene and Squirt
Age: 65
Primary condition: Schizoaffective disorder
Disclaimer at bottom of screen:
This was an individual patient’s experience. Results may vary.
TD, tardive dyskinesia.
Charlene:
He’s very spoiled. His name is Squirt…he loves me unconditionally. He has to be on a leash, and sometimes it took two, three tries before my hands would let me work the leash and get him hooked up.
My hands would shake, and I’d drop him sometimes…and then I’d be so mad at myself because I was afraid I hurt him…he never was angry with me when I dropped him, but I was angry with me that I dropped him. And I didn’t know TD was causing that…
Onscreen text:
After beginning treatment in 2020, Charlene experienced improvement in her involuntary movements. Charlene has now been on treatment for over 2 years.
Because of the reduced movements in Charlene’s hands, fingers, legs, and feet, Charlene is able to do activities with Squirt that were once more difficult.
Charlene:
Now I can hook the leash without trying hard…I can just hook it up. We can go for a walk…I don’t have to stop and take a break because…the movement in my feet causing me problems with my walking.
I don’t drop him…he trusts me more…now he’s not afraid of falling, he’s not afraid of me dropping him. He’s willing for me to pick him up and just lift him into my arms.
Onscreen text:
Charlene is currently taking AUSTEDO at 48 mg/day (24 mg BID).
The movements in Charlene’s hands, fingers, legs, and feet held her back from keeping up with Squirt, but AUSTEDO has helped her experience symptom improvement to move forward.
What could long-term results mean for your patients impacted by TD?
Learn more at AUSTEDOhcp.com
Onscreen ISI scroll:
INDICATIONS AND USAGE
AUSTEDO XR and AUSTEDO are indicated in adults for the treatment of chorea associated with Huntington’s disease and for the treatment of tardive dyskinesia.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease:
AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications:
AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
IMPORTANT SAFETY INFORMATION (Continued)
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism:
AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence:
Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia:
Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues:
Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions:
The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, on AUSTEDOhcp.com.
Onscreen text:
This was an individual patient’s experience. Results may vary.
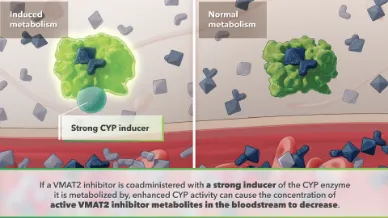
Metabolic Pathways
Visualizing the role of metabolic pathways in drug-drug interactions (DDIs).
Onscreen text:
CYP enzymes mediate the breakdown of active drug to inactive metabolites for clearance.
Together, CYP3A4/5 and CYP2D6 enzymes are involved in metabolizing ~75% drugs.
CYP enzymes play a key role in converting VMAT2 inhibitors into their active and inactive metabolites.
Certain drugs can act as strong CYP inhibitors or inducers, affecting the metabolism of coadministered medications…
…resulting in drug-drug interactions.
Inhibited metabolism
Inhibited metabolism results in elevated levels of active metabolites.
Elevated levels of active metabolites lead to increased drug potency and potential for adverse events.
Induced metabolism
Induced metabolism results in decreased levels of active metabolites.
Decreased levels of active metabolites diminish therapeutic effect.
For VMAT2 inhibitors, drug-drug interactions are determined by:
The CYP enzymes critical in metabolizing the VMAT2 inhibitor
AND
The metabolic profiles of the medications the patient is taking concomitantly (whether they are strong inhibitors or inducers)
To avoid drug-drug interactions associated with inhibited or induced metabolism, limiting VMAT2 inhibitor dose or avoiding use with strong inhibitors and inducers may be recommended.
When choosing a VMAT2 inhibitor, considering these drug-drug interactions is key.
VMAT2, vesicular monoamine transporter 2.

Treating TD while managing underlying conditions
See how Susan reduced her TD movements with AUSTEDO while maintaining her existing medication regimen.
Onscreen text:
TREATING TD WHILE MANAGING UNDERLYING CONDITIONS
SUSAN’S STORY
Age: 62
Primary conditions: Bipolar type II, seizure disorder
Disclaimer at bottom of screen:
This was an individual patient’s experience. Results may vary.
TD, tardive dyskinesia.
Susan:
My name is Susan Wheeler.
As a person who has tardive dyskinesia, I'm passionate to tell the story because there are treatments…
Onscreen text:
When Susan sought help for involuntary movements caused by antipsychotics, her psychiatrist prescribed a VMAT2 inhibitor.
Disclaimer on bottom of screen:
VMAT2, vesicular monoamine transporter 2.
But when Susan developed a seizure disorder, her TD treatment got more complicated.
Susan:
When I first received the diagnosis of TD and went to my doctor, I was prescribed another medication, and it worked for a while. But I then got another diagnosis of seizure disorder, and when I got that diagnosis, the new medications came on board and caused a conflict with that medication.
Onscreen text:
Susan is now on AUSTEDO XR because it can be taken with her seizure medication.
Onscreen Indication and Black Box Warning:
AUSTEDO XR® and AUSTEDO® are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington's disease.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Please see additional Important Safety Information at the end of this video.
Susan:
My doctor said that AUSTEDO was a good option for me, that it was a medication that would help my tardive dyskinesia but wouldn't interact with the medications I took for both my mental health and seizure disorders…
Onscreen text:
After Susan started on AUSTEDO, she noticed an improvement in her movements.
Susan:
AUSTEDO has helped me. I don't see the mouth tics anymore, which is a great relief to me, because I do a lot of public speaking, and sing with groups.
Even in retirement, you still want to feel good about yourself, and not have to deal with explaining your TD symptoms.
Onscreen text:
Learn more about how AUSTEDO XR can work with your patient’s concomitant medications at AUSTEDOhcp.com.
Disclaimer at bottom of screen:
Patient was compensated for her participation.
Onscreen ISI scroll:
INDICATIONS AND USAGE
AUSTEDO XR and AUSTEDO are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington's disease.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, at AUSTEDOhcp.com.

From diagnosis to movement improvement
Denise struggled with undiagnosed TD movements impacting her daily life and work as an NP. See how her journey to diagnosis and treatment with AUSTEDO XR improved her symptoms.
Onscreen text:
FROM DIAGNOSIS TO MOVEMENT IMPROVEMENT
DENISE’S TD STORY
Denise, FNP
Primary conditions: bipolar disorder, depression, anxiety, ADHD
Disclaimer on bottom of screen:
This was an individual patient’s experience. Results may vary.
ADHD, attention- deficit/hyperactivity disorder; TD, tardive dyskinesia.
Denise:
My name is Denise. I’m a Family Nurse Practitioner.
I see mental health patients. I see diabetes, hypertension, chronic kidney disease, all kinds of chronic disorders.
The most important thing about my job that I love is talking to my patients and communicating with them and getting to know them.
Onscreen text:
Denise had been taking antipsychotic medications for several years when she noticed involuntary movements in her jaw, hands, and feet—and so did her colleagues and patients.
Denise:
Gradually things just continued to get worse.
That feeling very much did affect my overall mental health. Most of my life was lived in a depression.
And I did worry about whether or not I'd be able to continue my work because it became harder and harder just to get through the day. And I didn't want to talk to people, and I didn't want to really do my job anymore.
Seeing patients became more of a duty instead of a passion anymore, because I was just trying to get through the day instead of taking time to acknowledge my patients.
Onscreen text:
Denise went 5 years without proper diagnosis or treatment while her movements continued to impact her daily activities...
…until her PCP recognized her movements as TD during a routine visit.
Disclaimer on bottom of screen:
PCP, primary care physician.
Denise:
The diagnosis of TD was more of a relief, because I knew that I had been mistreated the whole time and misdiagnosed for so long that when somebody said, "You actually have TD," it was like, maybe we can start over and actually get some treatment that works.
Onscreen Indication and Black Box Warning:
AUSTEDO XR® and AUSTEDO® are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington's disease.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression. Please see additional Important Safety Information at the end of this video.
Denise:
…As I started taking AUSTEDO XR, I noticed that within a couple of weeks that my feet…weren’t moving around everywhere. And then my jaw stopped moving as much…my speech returned to normal.
I'm on five different mental health medications to control my disorders. I didn't have to alter any of them.
…As I was being treated for my TD, my movements improved…my day-to-day life improved.
Onscreen text:
Thanks to accurate diagnosis and VMAT2 inhibitor education, Denise had finally found effective and tolerable TD treatment.
Disclaimer on bottom of screen:
Patient was compensated for her participation.
Onscreen text:
Learn more about how the right diagnosis—and the appropriate treatment—can make a difference for your patients like Denise at AUSTEDOhcp.com.
Onscreen ISI scroll:
INDICATIONS AND USAGE
AUSTEDO XR and AUSTEDO are indicated in adults for the treatment of tardive dyskinesia and for the treatment of chorea associated with Huntington's disease.
IMPORTANT SAFETY INFORMATION
Depression and Suicidality in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO can increase the risk of depression and suicidal thoughts and behavior (suicidality) in patients with Huntington’s disease. Balance the risks of depression and suicidality with the clinical need for treatment of chorea. Closely monitor patients for the emergence or worsening of depression, suicidality, or unusual changes in behavior. Inform patients, their caregivers, and families of the risk of depression and suicidality and instruct them to report behaviors of concern promptly to the treating physician. Exercise caution when treating patients with a history of depression or prior suicide attempts or ideation. AUSTEDO XR and AUSTEDO are contraindicated in patients who are suicidal, and in patients with untreated or inadequately treated depression.
Contraindications: AUSTEDO XR and AUSTEDO are contraindicated in patients with Huntington’s disease who are suicidal, or have untreated or inadequately treated depression. AUSTEDO XR and AUSTEDO are also contraindicated in: patients with hepatic impairment; patients taking reserpine or within 20 days of discontinuing reserpine; patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of discontinuing MAOI therapy; and patients taking tetrabenazine or valbenazine.
Clinical Worsening and Adverse Events in Patients with Huntington’s Disease: AUSTEDO XR and AUSTEDO may cause a worsening in mood, cognition, rigidity, and functional capacity. Prescribers should periodically re-evaluate the need for AUSTEDO XR or AUSTEDO in their patients by assessing the effect on chorea and possible adverse effects.
QTc Prolongation: AUSTEDO XR and AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO XR or AUSTEDO is administered within the recommended dosage range. AUSTEDO XR and AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Neuroleptic Malignant Syndrome (NMS), a potentially fatal symptom complex reported in association with drugs that reduce dopaminergic transmission, has been observed in patients receiving tetrabenazine. The risk may be increased by concomitant use of dopamine antagonists or antipsychotics. The management of NMS should include immediate discontinuation of AUSTEDO XR and AUSTEDO; intensive symptomatic treatment and medical monitoring; and treatment of any concomitant serious medical problems.
Akathisia, Agitation, and Restlessness: AUSTEDO XR and AUSTEDO may increase the risk of akathisia, agitation, and restlessness. The risk of akathisia may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops akathisia, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Parkinsonism: AUSTEDO XR and AUSTEDO may cause parkinsonism in patients with Huntington’s disease or tardive dyskinesia. Parkinsonism has also been observed with other VMAT2 inhibitors. The risk of parkinsonism may be increased by concomitant use of dopamine antagonists or antipsychotics. If a patient develops parkinsonism, the AUSTEDO XR or AUSTEDO dose should be reduced; some patients may require discontinuation of therapy.
Sedation and Somnolence: Sedation is a common dose-limiting adverse reaction of AUSTEDO XR and AUSTEDO. Patients should not perform activities requiring mental alertness, such as operating a motor vehicle or hazardous machinery, until they are on a maintenance dose of AUSTEDO XR or AUSTEDO and know how the drug affects them. Concomitant use of alcohol or other sedating drugs may have additive effects and worsen sedation and somnolence.
Hyperprolactinemia: Tetrabenazine elevates serum prolactin concentrations in humans. If there is a clinical suspicion of symptomatic hyperprolactinemia, appropriate laboratory testing should be done and consideration should be given to discontinuation of AUSTEDO XR and AUSTEDO.
Binding to Melanin-Containing Tissues: Deutetrabenazine or its metabolites bind to melanin-containing tissues and could accumulate in these tissues over time. Prescribers should be aware of the possibility of long-term ophthalmologic effects.
Common Adverse Reactions: The most common adverse reactions for AUSTEDO (>8% and greater than placebo) in a controlled clinical study in patients with Huntington’s disease were somnolence, diarrhea, dry mouth, and fatigue. The most common adverse reactions for AUSTEDO (4% and greater than placebo) in controlled clinical studies in patients with tardive dyskinesia were nasopharyngitis and insomnia. Adverse reactions with AUSTEDO XR extended-release tablets are expected to be similar to AUSTEDO tablets.
Please see full Prescribing Information, including Boxed Warning, at AUSTEDOhcp.com.

Sherland Shares Her Truth About TD
Learn how treatment with AUSTEDO over 4+ years got Sherland back to what she loved
APPROVED USES
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are prescription medicines that are used to treat:
the involuntary movements (chorea) of Huntington’s disease. AUSTEDO XR and AUSTEDO do not cure the cause of the involuntary movements, and it does not treat other symptoms of Huntington’s disease, such as problems with thinking or emotions.
movements in the face, tongue, or other body parts that cannot be controlled (tardive dyskinesia).
It is not known if AUSTEDO XR and AUSTEDO are safe and effective in children.
IMPORTANT SAFETY INFORMATION
AUSTEDO XR and AUSTEDO can cause serious side effects in people with Huntington’s disease, including: depression, suicidal thoughts, or suicidal actions. Do not start taking AUSTEDO XR or AUSTEDO if you are depressed (have untreated depression or depression that is not well controlled by medicine) or have suicidal thoughts. Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts or feelings. This is especially important when AUSTEDO XR or AUSTEDO is started and when the dose is changed. Call your healthcare provider right away if you become depressed, have unusual changes in mood or behavior, or have thoughts of suicide.
Individual results may vary.
Please see the Important Safety Information at the end of this video.
Once my involuntary movements were under control, I felt like I can live again. I can go outside again. I can be a part of the world again.
“Hello, my name is Sherland. I’m from Roxboro, North Carolina. I’m a published author. I edit novels. I am a great lover of literature and nature.”
Dear Sherland,
When this first started, you weren’t aware something was wrong… until your daughter asked why you were so fidgety. She told you that your mouth twitched when you talked and your fingers and feet constantly moved. When you realized you couldn’t control these movements, you grew self-conscious. TD transformed you from someone who loved conversing with strangers, to a timid individual who avoided family and friends because everyone asked when you had a stroke, not if you had a stroke, but when […]. TD left you feeling despondent and lonely.
My doctors told me that my involuntary movements…were side effects caused by my mental health medication. When I first found out that I had tardive dyskinesia, I did as much research as I could about it. It felt good to know that I had a name to put with what I was experiencing. And I felt like I wasn’t alone because other people were going through the same thing. The doctor told me when he recommended AUSTEDO, ‘I’ve heard of this treatment that I think might help you.’ AUSTEDO has allowed me to continue taking my mental health medication, because it’s really important. I suffer from major depression and bipolar. Before, I was just feeling hopeless. And after about two or three weeks I would say, I just started getting back out there because I had something to look forward to. I started seeing real progress with the involuntary movements. I have to say that it really made a difference in my life. I have confidence. I would tell them that there is a treatment, and it’s called AUSTEDO. And that you don’t have to feel hopeless like I did. You don’t have to struggle like I did. You can go to your doctor. You have a name, TD, to put with the symptoms. Ask about AUSTEDO and be proactive with your own health, because if you’re not, then who else will be? There’s no guarantees in life—but this is a good treatment plan for me.
APPROVED USE
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are prescription medicines that are used to treat:
the involuntary movements (chorea) of Huntington’s disease. AUSTEDO XR and AUSTEDO do not cure the cause of the involuntary movements, and it does not treat other symptoms of Huntington’s disease, such as problems with thinking or emotions.
movements in the face, tongue, or other body parts that cannot be controlled (tardive dyskinesia).
It is not known if AUSTEDO XR and AUSTEDO are safe and effective in children.
IMPORTANT SAFETY INFORMATION
AUSTEDO XR and AUSTEDO can cause serious side effects in people with Huntington’s disease, including: depression, suicidal thoughts, or suicidal actions. Do not start taking AUSTEDO XR or AUSTEDO if you are depressed (have untreated depression or depression that is not well controlled by medicine) or have suicidal thoughts. Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts or feelings. This is especially important when AUSTEDO XR or AUSTEDO is started and when the dose is changed. Call your healthcare provider right away if you become depressed, have unusual changes in mood or behavior, or have thoughts of suicide.
Do not take AUSTEDO XR or AUSTEDO if you:
have Huntington’s disease and are depressed or have thoughts of suicide.
have liver problems.
are taking reserpine. Do not take medicines that contain reserpine with AUSTEDO XR or AUSTEDO. If your healthcare provider plans to switch you from taking reserpine to AUSTEDO XR or AUSTEDO, you must wait at least 20 days after your last dose of reserpine before you start taking AUSTEDO XR or AUSTEDO.
are taking a monoamine oxidase inhibitor (MAOI) medicine. Do not take an MAOI within 14 days after you stop taking AUSTEDO XR or AUSTEDO. Do not start AUSTEDO XR or AUSTEDO if you stopped taking an MAOI in the last 14 days. Ask your healthcare provider or pharmacist if you are not sure.
are taking tetrabenazine. If your healthcare provider plans to switch you from tetrabenazine to AUSTEDO XR or AUSTEDO, take your first dose of AUSTEDO XR or AUSTEDO on the day after your last dose of tetrabenazine.
are taking valbenazine.
Other possible serious side effects include:
Irregular heartbeat (QT prolongation). AUSTEDO XR and AUSTEDO increases your chance of having certain changes in the electrical activity in your heart. These changes can lead to a dangerous abnormal heartbeat. Taking AUSTEDO XR or AUSTEDO with certain medicines may increase this chance.
Neuroleptic Malignant Syndrome. Call your healthcare provider right away and go to the nearest emergency room if you develop these signs and symptoms that do not have another obvious cause: high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, or increased sweating.
Restlessness. You may get a condition where you feel a strong urge to move. This is called akathisia.
Parkinsonism. Symptoms include: slight shaking, body stiffness, trouble moving, trouble keeping your balance, or falls.
Sleepiness (sedation) is a common side effect of AUSTEDO XR and AUSTEDO. While taking AUSTEDO XR or AUSTEDO, do not drive a car or operate dangerous machinery until you know how AUSTEDO XR or AUSTEDO affects you. Drinking alcohol and taking other drugs that may also cause sleepiness while you are taking AUSTEDO XR or AUSTEDO may increase any sleepiness caused by AUSTEDO XR and AUSTEDO.
The most common side effects of AUSTEDO in people with Huntington’s disease include sleepiness (sedation), diarrhea, tiredness, and dry mouth.
The most common side effects of AUSTEDO in people with tardive dyskinesia include inflammation of the nose and throat (nasopharyngitis) and problems sleeping (insomnia).
The most common side effects of AUSTEDO XR are expected to be similar to AUSTEDO in people with Huntington’s disease or tardive dyskinesia.
These are not all the possible side effects of AUSTEDO XR or AUSTEDO. Call your doctor for medical advice about side effects. You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please read the Medication Guide available at AUSTEDO.com, or by calling 1-800-887-8100.
TD Talks: Informative videos on TD evaluation and treatment

Amber Hoberg, PMHNP-BC, discusses the importance of assessing and managing TD.
Voiceover:
Welcome to TD Talks and today’s installment: The need to assess and manage tardive dyskinesia, or TD.
Amber Hoberg:
Hi, I’m Amber Hoberg. I’ve been a psychiatric nurse practitioner treating people with TD for more than 10 years.
As a known complication of antipsychotic drugs, TD affects about 500,000 patients in the United States.
Yet, many of these patients may not tell us about their symptoms or may minimize the impact that TD is having on their lives. It’s therefore critical that we do not make assumptions and do everything we can to uncover the true impact of TD. That way, we can ensure that patients receive the quality care they need.
So, if patients aren’t complaining about their TD symptoms, why are they still so important to assess and manage?
TD can have a profound impact on everyday life for these patients, including the way it affects their primary condition and treatment with antipsychotic drugs, or APDs. This often manifests in the cycle you see here.
Patients with TD can experience a significantly more severe and refractory course of their primary condition. This can include a poor response to care and an increased risk of relapse or readmission.
In addition, patients have said that involuntary movements affect their physical health and can make everyday activities a challenge.
Despite this, between 20 and 30 percent of patients may not be aware that they are experiencing TD symptoms. It is for these very important reasons that we need to be sure we’re assessing our patients for TD—even if they’re not bringing up the subject themselves.
Whether minor or very apparent, the symptoms of TD can affect every patient, and those who care for them, differently. That is why it is so important not only to talk to the patient, but to also involve the patient’s family or caregivers in your assessment.
For example, I often engage a patient’s spouse or partner to see the impact the symptoms might be having on their everyday lives and relationships at home.
We can start by asking about symptoms across these 3 categories: psychosocial, functional, and physical. These questions can help identify the presence of TD and evaluate the severity of each patient’s symptoms.
To see TD symptoms more clearly, you can ask your patient to perform activation maneuvers, as shown onscreen. Activation maneuvers can help reveal any underlying TD symptoms or highlight symptoms that may initially be less apparent. If any involuntary movements are noted, a thorough workup should be done.
Although asking patients about the impact of symptoms on their life is the first step in our evaluation, it is also important to consider the objective severity of TD symptoms and how they may affect functioning.
Therefore, it is best practice to also use standardized measures such as the AIMS scale. By using the AIMS scale, we have a consistent way to evaluate patients’ TD symptoms and response to management over time.
Remember, it is not just the more severe scores that require further evaluation—any score above zero should trigger a deeper qualitative assessment on how those functions may be affecting their daily life.
AIMS can be performed in both in-person or telehealth settings. However, virtual assessments should be intermixed with live exams when possible.
The APA recommends clinical assessment of abnormal involuntary movements prior to initiating APD therapy and at follow-up visits. The exact frequency of AIMS follow-up is driven by APD history and level of risk.
If assessments are scheduled virtually, there are a few considerations to keep in mind.
Be sure to work out the appropriate camera angle with your patient prior to initiating the exam. I recommend activating your patients by mimicking their maneuvers on your end of the screen—so make sure they can see you, too.
If you cannot see your patient clearly onscreen, you may need to rely on patient commentary or a caregiver’s observation to evaluate certain body locations. If the platform doesn’t work at all, request to switch to a different program so you’re able to view your patient more clearly.
As rigidity cannot be formally assessed remotely, observe the arms while the patient is walking. Absence of arm swing can be a clinical sign of rigidity.
Lastly, be aware of the limitations that may be encountered during the assessment, including the inability to observe lower limbs, midline structures, and upper limbs. Inability to assess rigidity associated with parkinsonism; and technological limitations that may affect the test.
In summary, it’s critical to identify and address TD in order to minimize the effects that it can have on patients’ well-being.
Do so by leveraging established assessment criteria, and ask further questions to reveal the true impact that their symptoms may be having on their life.
Remember, by assessing the impact of TD and managing symptoms, you can make a tremendous difference in a patient’s life.
Thanks for watching this installment of TD Talks.
TD virtual assessments, demonstrated by Dr. Arvinder Walia

A long virtual assessment, with full view of the patient’s body
Arvinder Walia:
Hi, good afternoon, Carol. How are you?
Carol:
Hi Dr. Walia, I’m doing great.
Arvinder Walia:
Good to hear that.
So, since our last visit, everything is coming along all right?
Carol:
Yes, sir, it sure is.
Arvinder Walia:
Okay.
Okay, Carol. One of the medicines that you are on, uh, one of the things for that medicine that we have to watch out for is a certain, uh, kind of side effect, which can affect movement.
So, I’m going to ask you a few questions and prompt you to do a few things here over the next couple minutes or so, uh, to make sure we are taking care of that aspect. So, with that being said, here's my first question:
Have you or your family or loved one noticed any sort of abnormal movement in your face area, on your lips, mouth, or tongue? In your arms, um, legs, anywhere?
Carol:
No, sir.
Arvinder Walia:
Okay. Okay.
Okay. Now before we start the exam, um, want to make sure, is there anything in your mouth, like chewing gum?
Carol:
No.
Arvinder Walia:
Do you wear dentures?
Carol:
No, I do not.
Arvinder Walia:
Okay, okay. All right. So, what I’d like you to do is sit comfortably in that chair, uh, make sure your arms are kind of like this, not using the armrest, just resting on your legs. And what I’m going to do is observe your—just do an observation.
Okay, all right. So now, what I want you to do is if you can see me, start, uh, tapping your finger with the thumb of the right hand. Keep doing that.
Switch it over to the left. Okay, we’re good.
Now, open your mouth, wide open. Yep. Now you can close it, good. Can you do it one more time for me? That’s good.
So, next time around I’m going to ask you to open your mouth, but this time stick your tongue out. So, open your mouth and tongue out. Good. You can close. We’re going to repeat it one more time. Open your mouth and stick your tongue out. Perfect.
Okay, so, back again to the finger tap. Now open your mouth, keep tapping the finger, stick your tongue out. All right, that’s good. That’s good. You doing OK?
Carol:
Yes, I’m doing fine.
Arvinder Walia:
Okay. So, the next thing I’m going to ask you to do, is cause I want to see the whole body, so the way the camera is right now, I can’t. I want you to move to that corner to the left of you, but before that, I want to make sure that if you are wearing shoes and socks, can you take the shoes and socks off?
Carol:
Yes, sir.
Arvinder Walia:
Okay. Then, now get up and walk over to that corner please, and face the camera. That’s good. That’s good, just be comfortable.
Now, stretch your arms, if you can see me, all the way out. Okay, that’s good. You can rest.
Now I’d like to have a side view, so if you can face the wall, give enough space there, yeah just for right now, just be comfortable.
Now do the same thing with stretching your arms far out. Okay. We’re good. You can be comfortable back. Come back, have a seat, thank you again.
Okay. Uh, oh, I’ll have to ask you to get up, because I forgot to ask you to walk a little bit. So, go back in that corner. Walk towards the camera, turn around, go back towards the wall. Okay, perfect. You can come back and have a seat.
Carol:
OK, thank you.
Arvinder Walia:
Thank you.
Okay, so, the question is, I noticed some gait issues there. Are you having any problems with balance?
Carol:
I’m not having balance problems. My hips are hurting, because of osteoarthritis.
Arvinder Walia:
Okay, okay. Are you having any stiffness in any muscles, uh, throughout the body—muscle stiffness?
Carol:
No muscle stiffness.
Arvinder Walia:
Okay, okay.
All right, then. That’s pretty much all the questions that I had, uh, for you. That should be the end of our exam. Thank you again, Carol.
Carol:
Thank you, Dr. Walia.

A short virtual assessment, without full view of the patient’s body
Arvinder Walia:
Good morning, Angie.
Angie:
Good morning, Dr. Walia.
Arvinder Walia:
How are you doing?
Angie:
I’m doing well, thank you.
Arvinder Walia:
Good, good. So, everything since our last visit, you feel has been going well? Your medicines are working okay?
Angie:
Yes, very good. No ups or downs and feeling very well.
Arvinder Walia:
Good. Good.
Uh, Angie, uh, one of the medicines that you take, uh, we have to watch out for certain side effects of that, uh, medicine.
So, what we have to do is periodically do a certain exam, uh, at, some regular intervals. So, I am going to be running you through that.
Um, it is going to be brief questions. Um okay, with that being said, here is my first question for you:
Have you or your family members noticed any abnormal movements either in your face, your arms, hands, or legs?
Angie:
No, not recently.
Arvinder Walia:
Okay. Okay. Well, let’s move further then.
Now before I start, um, quick question, anything in your mouth right now like a chewing gum or anything you have in your mouth?
Angie:
No.
Arvinder Walia:
Okay. Any dentures, uh, you wear…
Angie:
I have permanent implants.
Arvinder Walia:
And right now, they are in good shape, right?
Angie:
Yes.
Arvinder Walia:
Okay, all right. So, for the purposes of this exam, I’ll ask you if you can take your, uh, glasses off…
Now rest comfortably in the chair. Just make sure you don’t use the armrest for your arm, just let your arms rest on your legs.
Just be comfortable and I’m just going to be observing you for a few minutes here.
Okay. So, now what I want you to do is tap your right finger and thumb as I show you…
Start tapping it fast, keep doing it.
Move to the left, keep doing it. Okay, that’s good, you can rest back.
Now open your mouth wide open, that’s good. You can close it now. I’m going to ask you to open it again one more time, the same way. Okay, you can close it now.
So, I’m going to ask you to open your mouth again, but this go-around I’m going to ask you to stick your tongue out.
Good. You can close it. Open it one more time and stick your tongue out. Okay, you can close it.
We’re going back to the finger tap like we were doing, fast, now open your mouth as you are tapping it, yeah… stick your tongue out, keep tapping it, stick your tongue out. Okay, that’s good. You can rest.
Now, have you noticed any abnormal movement in your hands or in your arms?
Angie:
No, not lately.
Arvinder Walia:
Okay. Anything you’ve noticed like any abnormal movements in either your shoulders or your hips?
Angie:
No.
Arvinder Walia:
Any sort of strange abnormal movements in your legs, or your feet, or toes?
Angie:
No.
Arvinder Walia:
Okay, okay. Have you noticed any problems, uh, with your balance or gait?
Angie:
Yes, I do have some problems with my balance, um, I seem to sort of stumble to the right and then I have, um, trouble keeping my balance, like, on a bicycle and in restricted ways like that, I have a hard time keeping my balance.
Arvinder Walia:
Okay. Any stiffness in any part of the body or muscles that you’ve noticed?
Angie:
Yes, in my shoulders.
Arvinder Walia:
Tell me a little more about it.
Angie:
It seems to wax and wane. Um, I will have stiffness for several days at a time, and then it will go away, and then it will come back, and I will have it again for several days and then it will go away.
Arvinder Walia:
Is there any relationship in terms of timing? Uh, in other words, like stiffness comes, uh, comes in an hour or two after you take any of your medicines?
Angie:
No, not at all.
Arvinder Walia:
Okay. Okay. What about if there is any slowness in movements that develop, or sort of a fatigue, and slowness if you repeatedly continue to use a certain, uh, certain group of muscles?
Angie:
Yes, again, it is on my right side, and it’s in my arm a lot, and it just gets to be, where I know I’m not 100%.
Arvinder Walia:
Okay. Well, well, thank you Angie, uh, sort of, uh, ends our exam.
Differentiating tardive dyskinesia (TD) vs drug-induced parkinsonism (DIP)

Unraveling the Neurobiology of TD and DIP
Andrew Cutler, MD, uses unique graphics to illustrate the neurobiological distinctions between TD and DIP that set these 2 disorders apart, and explains how inappropriate treatment may worsen symptoms of TD.
Andrew Cutler:
Hello, I’m Dr Andrew Cutler, Chief Medical Officer at the Neuroscience Education Institute in Carlsbad, California, and a Clinical Associate Professor of Psychiatry at SUNY Upstate Medical University in Syracuse, New York.
Today, on behalf of Teva Pharmaceuticals, I’ll speak about the importance of differentiating tardive dyskinesia or TD, and drug-induced parkinsonism or DIP. We’ll take a look at how these disorders affect dopamine signaling to better understand why anticholinergics should not be used in patients with TD.
TD and DIP are common movement disorders caused by exposure to antipsychotic drugs.
It is unfortunate that the term extrapyramidal symptoms, or EPS, has historically been used to describe any drug-induced movement disorder, because it is a nonspecific term.
TD and DIP are both included under the umbrella of EPS and are often treated indiscriminately with anticholinergic medications. However, this concept is outdated. Today, we know these disorders are distinct and the use of anticholinergics for all drug-induced movements is inappropriate.
TD and DIP have different underlying causes, opposite clinical presentations, and very different treatments. In fact, treatment for 1 movement disorder may worsen the other.
Treatment guidelines from the American Psychiatric Association or APA and the DSM-5-TR provide recommendations for TD and DIP.
The APA guidelines for the treatment of schizophrenia recommend treating TD with a vesicular monoamine transporter 2, or VMAT2, inhibitor if symptoms have an impact on the patient, regardless of severity.
The DSM-5 cautions that symptoms of TD tend to be worsened by anticholinergic medications, such as benztropine.
Although anticholinergics are indicated for the treatment of DIP, the APA cautions that these agents can result in multiple difficulties for patients, including impaired cognition, diminished quality of life, and significant health complications.
The APA advises that it is important to keep in mind the total anticholinergic burden when selecting a medication for DIP. For this reason, antiparkinsonian medications are not typically administered on a prophylactic basis.
If an anticholinergic is used, it is important to adjust the medication to the lowest dose that can treat the symptoms, in order to minimize side effects. In addition, they should be used for the shortest time necessary.
Despite warnings about anticholinergic use, about 40% of psychiatry providers indicated they would initiate benztropine to treat, as well as to prevent, TD. Clearly the distinction between TD and DIP is misunderstood among the greater healthcare community.
The causes of TD and DIP can help explain their treatment. I hope that after watching this video you will have a better understanding of their biologic mechanisms, clinical symptoms, and appropriate treatments.
Patients with DIP display slowness of movement, or bradykinesia. I’ll demonstrate how this slowness, or as we see here, frozen appearance correlates with decreased dopamine.
In contrast, patients with TD have an excess of abnormal, irregular movements.
I’ll start with a refresher on the neurobiology of normal movement and then explain how signaling is altered in patients with DIP versus TD.
We will also review how anticholinergics resolve symptoms related to the mechanisms of DIP versus TD.
Movement is controlled by dopamine signaling in the basal ganglia. We’ll be discussing a presynaptic neuron that receives the signal to initiate movement and a postsynaptic neuron that transmits the signal to produce movement.
Here we can see that VMAT2 has transported dopamine into vesicles,
…where it waits for the signal to be released.
When the signal is received by the presynaptic neuron to initiate movement, the vesicles fuse with the presynaptic membrane and release dopamine into the synapse.
Dopamine binds to postsynaptic receptors…
and a signal is transmitted to initiate movement.
In DIP, antipsychotic drugs transiently block postsynaptic dopamine receptors.
When dopamine is now released, the antipsychotic drug is bound to dopamine receptors, and the blockade results in reduced dopamine signaling in the post synaptic neuron.
Reduced dopamine signaling results in bradykinesia, or slowness of movement.
Since the mechanism of DIP occurs when antipsychotics are first started, it makes sense that the clinical signs of DIP occur early, within a few weeks to months of starting or increasing the dosage of an antipsychotic.
The exact pathophysiology of TD is not fully known. However, it is thought that chronic blockade of dopamine receptors by antipsychotics results in the upregulation, or a hypersensitivity, of dopamine receptors.
This upregulation results in increased dopamine signaling, which manifests as the excessive movements that are characteristic of TD, which is a possible explanation for why DIP is a risk factor for TD.
In TD, a chronic blockade of dopamine receptors by antipsychotics results in an upregulation, or hypersensitivity, of dopamine receptors. So, TD develops after using an antipsychotic for at least a few months, typically years.
Some elderly or high-risk patients may develop symptoms in a shorter period.
Discontinuing the antipsychotic may improve or resolve the symptoms associated with DIP.
In contrast, reducing the dose or discontinuing an antipsychotic may fail to improve the symptoms of TD or might even induce withdrawal dyskinesia.
Anticholinergic agents like benztropine act to promote dopamine signaling. This can help alleviate the bradykinesia associated with DIP, but it is the exact opposite of what we want to do with TD.
If we add more dopamine to the postsynaptic neuron in TD, the already upregulated receptors may transmit an even greater number of signals. This could result in an even further increase in abnormal movements.
As mentioned previously, guidelines recommend VMAT2 inhibitors for adults with TD.
It is believed that VMAT2 inhibitors reduce the amount of dopamine packaged into presynaptic vesicles, which may reduce the amount of dopamine released at the synapse and reduce TD movements.
If we use a VMAT2 inhibitor in a patient with DIP, it could worsen symptoms, as reduced levels of dopamine being released into the synapse can reduce the already decreased dopamine signaling and further slow the patient’s movement.
Recognizing the different neurobiology and clinical presentation between TD and DIP can help clinicians choose appropriate treatment.
I hope this video helped you understand these differences and will encourage you to apply this knowledge to help your patients.

Differentiating the Clinical Presentations of TD and DIP
Rajeev Kumar, MD, uses videos of real patients with TD and DIP to showcase the clear clinical differences between these 2 movement disorders. He explains the risk of misdiagnosis in TD and how it may lead to improper, and possibly harmful, treatment.
Rajeev Kumar:
Hello. I’m Dr Rajeev Kumar, the medical director of the Rocky Mountain Movement Disorders Center and the Huntington’s Disease Society of America, Center of Excellence in Englewood, CO. I’ve been working with patients with tardive dyskinesia, or TD, for more than 20 years.
Today, on behalf of Teva, I'll be speaking about clinical symptoms and treatment of TD and its differentiation from drug-induced parkinsonism, or DIP. Movement disorders in patients taking antipsychotics are common and up to one-third of them will develop either TD, DIP, or both.
I’ll be focusing on how these 2 disorders may be misdiagnosed, and how treating a patient with TD for DIP could have adverse consequences for the patient. I’ll also speak about how the opposite is true as well.
Tardive dyskinesia and drug-induced parkinsonism are both very common movement disorders, and both are caused by dopamine receptor blocking medications, like antipsychotics.
However, they present differently, differ in their pathophysiology with respect to dopaminergic signaling, and have different treatments. In fact, treatment for one disorder may worsen the other.
The term EPS, or extrapyramidal symptoms, has been used in psychiatry for many years, and this term has been propagated by the labeling of medications by the FDA.
Historically, EPS has been used to describe all drug-induced movement disorders experienced by patients taking antipsychotic medications. As a result, there’s been the indiscriminate treatment of both disorders with anticholinergic medications.
We now know that the term EPS is outdated. It’s very important to differentiate between the disorders because they merit different treatments.
The use of antipsychotics has markedly increased over the past 2 decades due to expanded indications for use in mood disorders, such as depression and bipolar disorder.
The data suggests that in 2022, 8.7 million Americans received prescriptions for an antipsychotic and, as a result, we estimate there are at least 785,000 individuals who have TD in the United States.
Now that's a shocking number, and even more surprisingly, at least 85% of patients have been misdiagnosed or remain undiagnosed.
Many of these individuals, who have been misdiagnosed, have been labeled as having EPS, and many of them, in fact, have DIP.
So, this means that about 15% of patients have been diagnosed and only 6% of individuals have been treated with a VMAT2 inhibitor.
If we look at US healthcare claims data of those individuals who have been diagnosed with TD, about 36% of individuals are receiving benztropine with about three-quarters of these individuals having received benztropine for more than 3 months, and about a-third having been treated for greater than a year.
And if we look at online surveys of psychiatry providers, about 40% of individuals indicate that they would actually initiate benztropine to treat TD, which is an unfortunate failure of our ability to educate these providers.
So, for patients suffering from TD, the lack of an accurate diagnosis can lead to mistrust and frustration. And of course, for patients that have been misdiagnosed, the wrong therapy can result in a worsening of their symptoms.
As a result, patients can fail to seek care because of their frustration and may not follow instructions correctly.
Let's talk a little bit about the underlying mechanisms of disease for both TD and DIP, and then we'll review some videos that could help one make appropriate diagnostic and treatment decisions when seeing patients with drug-induced movement disorders.
First, let's talk about the time course of TD and DIP. DIP occurs due to acute blockade of predominately dopamine D2 receptors.
Now, because of this blockade, we get a reduction in postsynaptic dopamine signaling.
Tremor, bradykinesia, rigidity, and impairment of gait may become apparent within the first few weeks or first few months of starting or increasing the dose of the antipsychotic.
In tardive dyskinesia, the term “tardive” means delayed. Chronic blockade of dopamine receptors by antipsychotics results in delayed upregulation, or super sensitivity, of these postsynaptic dopamine receptors. So, TD usually develops after months or years of exposure to antipsychotic medications.
Let's review the clinical features of DIP and TD and see how they're differentiated when we examine patients.
The degree of movements is one of the first things I look for.
This patient with DIP has a masked face with reduced facial expression and reduced blink rate, which is different from the typical normal level or increased level of facial movement we see in TD.
This patient also has mildly flexed posture, reduced arm swing, reduced stride length, reduced speed, and multi-step turns, all of which are characteristic of parkinsonism.
The nature of movements in DIP and TD are also different. When patients with DIP have abnormal movements, it is rhythmic.
This elderly gentleman has typical 4 to 5 Hertz resting tremor in the left hand, and it is not uncommon for the tremor to improve during sustained action and posture, but it may persist.
You can see this with the postural tremor in this gentleman's right hand.
And in fact, if we allow the jaw to hang down, he has synchronous tremor of his jaw.
TD is characterized by excessive movement or hyperkinesia.
This patient has continuous movements of the neck, jaw, and face. We can see she has quivering movements of the lips. In addition, she has excessive right shoulder elevation and right head tilt.
This patient has undulating movements of the lips, occasional tongue protrusion, and excessive blinking.
This younger woman has excessive lip pursing, platysma contraction, frontalis activation, and eyebrow elevation.
This patient has tongue movements that are unique. They go back and forth and then to one side of his mouth. He has very severe back and forth movements of his tongue, which are unpredictable, and, in fact, you can see that he has involuntary jaw closing and briefly bites his tongue.
This patient’s TD movements are irregular, jerky, and unpredictable. This patient has severe generalized choreiform movements, with piano playing movements in the fingers and the toes, body rocking, and facial dyskinesias with grimacing, and we can see the protrusion of his tongue within the mouth.
Let's look at the upper extremity muscle tone, which is a component of the AIMS examination.
First, we examine rigidity at the level of the wrist by isolating the wrist and passively moving it. Then we examine the tone at the level of the elbow.
Now, if you do not detect increased tone at rest, have the patient open and close the other hand, while we examine the tone again at the level of the wrist and the elbow.
We should do this for each side of the body independently. As you can see, I can easily move this TD patient’s arm at the level of the wrist and the elbow without resistance, both with and without activation of the contralateral hand.
Performing the same maneuvers on this man with parkinsonism, you can see that I don't have difficulty moving his wrist, but there is a resistance that occurs when I attempt to flex and extend his elbow.
Now, this is something you predominantly feel, but here you can almost see that I'm having some difficulty extending his elbow.
To make an accurate diagnosis of TD, it is essential that we are able to carefully assess and differentiate TD from DIP.
Because if one makes a misdiagnosis and prescribes an anticholinergic to a patient with TD, you may exacerbate the hyperkinetic movements by increasing dopaminergic signaling.
On the other hand, if you prescribe a dopamine-depleting VMAT2 inhibitor to a patient with DIP, you further reduce the patients already low level of dopamine, and this can worsen the patients’ features of parkinsonism.
So, it's important to differentiate TD and DIP by careful observation and examination of the patient. With the correct diagnosis, we can render the correct treatment.
Thank you for watching, and I hope what I’ve shared today will help you in the assessment of your patients with movement disorders who are taking antipsychotic medications.

Deciphering the Diagnostic Landscape: TD and DIP
Craig Chepke, MD, offers a broad overview of the distinctions between TD and DIP and the importance of making correct diagnoses.
Craig Chepke:
Hi, I’m Dr. Craig Chepke, adjunct professor of psychiatry for Atrium Health and medical director of Excel Psychiatric Associates in Huntersville, North Carolina.
Today, on behalf of Teva, I’ll talk about the importance of differentiating tardive dyskinesia, or TD, and drug-induced parkinsonism, or DIP. The American Psychiatric Association, or APA, and the DSM-5-TR, provide distinct treatment recommendations for TD and DIP, but there is a disconnect between the guidelines and how patients are being managed in the real world. Data indicate that anticholinergics are being used to treat TD, but this is not an effective treatment strategy and can have a number of detrimental consequences for patients.
Approximately one-third of patients taking antipsychotic drugs, or APDs, will experience TD, DIP, or both.
In the past, TD and DIP were both described by the term “EPS,” or extrapyramidal symptoms, and as such, patients with both disorders were managed with anticholinergics.
However, the concept of EPS is outdated. We now understand that TD and DIP have different underlying causes, opposite clinical presentations and, therefore, very different treatments.
Unfortunately, the concept of EPS is so well entrenched in psychiatry, and many providers still don’t recognize that TD is distinct from DIP.
It’s critical to differentiate patients with TD from patients with DIP, because treatment for one disorder may exacerbate the other disorder.
The APA Practice Guideline for the Treatment of Schizophrenia and the DSM-5-TR guidelines provide guidance for the treatment of each disorder, as well as several precautions that should be made when considering anticholinergic medications.
VMAT2 inhibitors, which are approved for TD, are recommended if symptoms have an impact on the patient.
In contrast, symptoms of TD tend to be worsened by anticholinergic medications, such as benztropine.
Anticholinergics are indicated for the treatment of DIP; however, their use has been linked to impaired quality of life, impaired cognition, and significant health complications.
Guidelines recommend considering the anticholinergic burden before prescribing a medication in this class. Many medications have anticholinergic properties, and for this reason, anticholinergics are not typically administered prophylactically when starting an antipsychotic.
If an anticholinergic is used, it should be adjusted to the lowest dose possible for the shortest time necessary.
Despite the recommendations regarding the use of anticholinergics, a survey of providers indicated that fewer than 40% are familiar with the 2020 APA Practice Guideline for the Treatment of Schizophrenia.
In the United States, TD affects approximately 785,000 patients.
However, only about 15% of these patients have received a formal diagnosis…
and less than 6% have been treated with a VMAT2 inhibitor.
So, this leaves as many as 85% of patients with TD either being missed, or possibly, misdiagnosed with DIP.
In addition, approximately 40% of psychiatric providers stated they use benztropine to prevent or treat TD.
In a separate analysis of patients who were diagnosed with TD, 36% were receiving benztropine. Additional analyses from the same data set indicated that, of those patients with TD who were taking benztropine, about 75% of them remained on treatment for over 3 months, and 35% were treated for over a year.
Training and education to reduce use of benztropine in TD is needed because clinical symptoms can be tied to the relative changes in dopamine signaling that can occur. I’ll be reviewing the mechanisms underlying TD and DIP to explain why treating patients for TD with anticholinergics can worsen symptoms of TD. Recognizing the unique symptoms and neurobiology of both disorders may help clinicians align with the treatment guidelines.
The time that it takes for symptoms of these disorders to present after use of antipsychotic drugs differs along with their clinical presentations.
Parkinsonian symptoms generally begin within a few weeks to a few months of starting or increasing the dosage of an antipsychotic drug.
DIP occurs due to an acute blockade of dopamine receptors, which leads to a reduction in postsynaptic dopamine signaling.
On the other hand, in TD, the chronic blockade of dopamine D2 receptors by antipsychotics result in an upregulation and hypersensitivity of dopamine receptors.
As such, TD develops after using an antipsychotic drug for at least a few months, but more typically over a few years. By DSM-5-TR criteria, a diagnosis of TD requires at least 3 months of exposure to antipsychotics for adults, but as little as 1 month for elderly patients.
The symptoms of DIP are correlated with a decrease in dopamine signaling, whereas the symptoms of TD are correlated with a relative increase in dopamine signaling. We generally see reduced movement in patients with DIP vs excessive movement in TD.
DIP is characterized by a paucity or slowness of movement. The person in this video demonstrates a masked face consistent with DIP; she appears to be staring and rarely blinks.
In contrast, a person with TD has movements that are excessive and often continuous.
This patient has an increased rate of blinking. Instead of a frozen look, she is puckering her lips repeatedly.
The nature of movements, whether hyperkinetic or hypokinetic, is one of the most important features to help me differentiate TD from DIP.
While DIP is generally associated with a paucity of movement, patients with DIP may sometimes have a tremor; however, the nature of that tremor is different from the movements of TD.
The patient has a parkinsonian tremor in his left hand, which occurs in a predictable and rhythmic fashion.
In contrast, the movements of TD are unpredictable, irregular, and sometimes even jerky. This patient has frequent involuntary movements, especially notable in his fingers and toes.
Evaluating the patient’s muscle tone is also helpful in differentiating TD from DIP.
DIP may be accompanied by rigidity that can be felt and confirmed in a physical exam.
In contrast, patients with TD have normal muscle tone.
Discontinuing the APD may improve or resolve the symptoms associated with DIP.
But if left untreated, TD is typically persistent and irreversible. APD reduction or withdrawal may fail to improve the symptoms of TD or might even induce withdrawal dyskinesia.
Based on the differences between TD and DIP, we must consider which treatment approach can best address this specific condition.
Anticholinergics are indicated for the treatment of DIP. However, the guidelines and the label for benztropine, which is a commonly used anticholinergic, warn that these agents do not alleviate the symptoms of TD and in some cases, may even aggravate them.
VMAT2 inhibitors are recommended for adults with TD but they do have the potential to worsen DIP.
Contrary to recommendations made in treatment guidelines, anticholinergics are being overprescribed in patients with TD, and only a fraction of patients with TD are being treated with a VMAT2 inhibitor.
The APA and DSM-5-TR guidelines reinforce that TD and DIP are 2 very different disorders with different proposed pathophysiologies. As such, it’s critical that we make the right diagnosis so we can select the right treatment approaches for our patients.
I hope this video has provided a helpful overview of these two disorders and will be useful in the clinical management of your patients.
For more videos, visit the YouTube page.
For more videos, visit the YouTube page.
VMAT2, vesicular monoamine transporter 2.
REFERENCE: 1. Solmi M, Pigato G, Kane JM, Correll CU. Treatment of tardive dyskinesia with VMAT-2 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:1215-1238.