Use AIMS to monitor for and assess tardive dyskinesia (TD) symptoms1
Guidelines for screening and routine monitoring of patients on antipsychotic drugs
The American Psychiatric Association (APA) recommends1:
- Clinical assessment for TD at each visit
- Assessment with a structured instrument, such as AIMS (Abnormal Involuntary Movement Scale):
- If a new onset or an exacerbation of preexisting movements is
detected - At least every 6 months in patients at high risk of TD
- At least every 12 months in other patients
AIMS is the standard structured assessment for the initial screening and the routine monitoring of TD symptoms2-4
The AIMS evaluates symptom severity across 12 items
- Items 1-7 assess the severity of involuntary movements across body regions
- Item 8 is based on the highest single score of items 1-7 and may be used independently as an indication of overall severity
- Items 9 and 10 assess the impact of TD and may be useful in clinical decision-making
- Items 11 and 12 assess dental issues
Abnormal Involuntary Movement Scale
Scoring items 1-74,5
Each of the first 7 items is scored on a 0 to 4 scale, rated as:
- 0: Not present,
- 1:Minimal, may be extreme normal (abnormal movements occur infrequently and/or are difficult to detect),
- 2:Mild (abnormal movements occur infrequently and are easy to detect),
- 3:Moderate (abnormal movements occur frequently and are easy to detect),
or - 4:Severe (abnormal movements occur almost continuously and/or are of extreme intensity)
The sum of items 1-7 is the AIMS total score. This may range from 0 to 28. A decrease in score between visits indicates an improvement in symptoms.
Use the TD Estimator Tool to see how many patients in your practice may have TD
Semistructured assessments may be used in between formal AIMS assessments1,6
Semistructured assessments may include1,6:
- Patient recognition of abnormal movements when reviewing antipsychotic side effects
- Visual observations during examination
- Patient or care partner report of abnormal movements
- Patient complaints of movements being distressful or interfering with daily life
If the semistructured assessment reveals abnormal movements, a full AIMS assessment should be performed.
Screening for abnormal movements can be accomplished by observing cognitive and physical activation maneuvers7,8

Ask the patient to:
Extend arms in front of the body and count backward from 100
Observe:
Hands and arms
- Cognitive activation maneuvers help to distract the patient and reveal abnormal movements8-11
- These mental tasks should be adapted based on the patient’s cognitive abilities and knowledge
- Additional cognitive activation maneuvers can include counting backward by serial 7s (eg, 100, 93, 86), reciting the alphabet backward, and naming the months in reverse order

Ask the patient to7,8:
Hold mouth open and stick tongue out while tapping thumb to forefinger with both hands
Observe:
Tongue and face

Ask the patient to2,8:
Walk in a straight line with usual gait and posture
Observe:
Hands, arms, tongue, and face
Assessing TD quickly, efficiently, and accurately in the telehealth setting9,12,13
Benefits of evaluating patients in the telehealth setting include14-16:
- Observing patients in their own environments
- Bringing family members into the conversation
- Reducing no-show rates
Before the telehealth visit9,17
Recommend that a care partner be present during the visit to assist with camera positioning and other aspects of the examination.
Ask the patient or care partner to make sure they have the following:
- A straight-back chair with no arms
- Enough space to:
- Sit in a chair for review of movements
- Walk back and forth while being observed
- Appropriate lighting
- Bandwidth to ensure good resolution to see movements
During the telehealth visit
The use of AIMS in the telehealth setting has been validated in a well-constructed reliability study.13,18
- Assessing 6 of 7 AIMS items can be done with the patient seated, and all 7 AIMS items may be assessed with additional camera positioning3,12
- Ask questions to uncover TD and determine its impact on the patient’s daily life12,19
- To assess rigidity, ask the patient if they experience stiffness and observe the patient while they are walking. An absence of arm swing is a clinical sign that there may be rigidity15,17
Watch Dr. Arvinder Walia demonstrate how to conduct TD virtual assessments
Watch: Long Virtual Assessment
A virtual assessment with full view of the patient’s body.
Arvinder Walia:
Hi, good afternoon, Carol. How are you?
Carol:
Hi Dr. Walia, I’m doing great.
Arvinder Walia:
Good to hear that.
So, since our last visit, everything is coming along all right?
Carol:
Yes, sir, it sure is.
Arvinder Walia:
Okay.
Okay, Carol. One of the medicines that you are on, uh, one of the things for that medicine that we have to watch out for is a certain, uh, kind of side effect, which can affect movement.
So, I’m going to ask you a few questions and prompt you to do a few things here over the next couple minutes or so, uh, to make sure we are taking care of that aspect. So, with that being said, here's my first question:
Have you or your family or loved one noticed any sort of abnormal movement in your face area, on your lips, mouth, or tongue? In your arms, um, legs, anywhere?
Carol:
No, sir.
Arvinder Walia:
Okay. Okay.
Okay. Now before we start the exam, um, want to make sure, is there anything in your mouth, like chewing gum?
Carol:
No.
Arvinder Walia:
Do you wear dentures?
Carol:
No, I do not.
Arvinder Walia:
Okay, okay. All right. So, what I’d like you to do is sit comfortably in that chair, uh, make sure your arms are kind of like this, not using the armrest, just resting on your legs. And what I’m going to do is observe your—just do an observation.
Okay, all right. So now, what I want you to do is if you can see me, start, uh, tapping your finger with the thumb of the right hand. Keep doing that.
Switch it over to the left. Okay, we’re good.
Now, open your mouth, wide open. Yep. Now you can close it, good. Can you do it one more time for me? That’s good.
So, next time around I’m going to ask you to open your mouth, but this time stick your tongue out. So, open your mouth and tongue out. Good. You can close. We’re going to repeat it one more time. Open your mouth and stick your tongue out. Perfect.
Okay, so, back again to the finger tap. Now open your mouth, keep tapping the finger, stick your tongue out. All right, that’s good. That’s good. You doing OK?
Carol:
Yes, I’m doing fine.
Arvinder Walia:
Okay. So, the next thing I’m going to ask you to do, is cause I want to see the whole body, so the way the camera is right now, I can’t. I want you to move to that corner to the left of you, but before that, I want to make sure that if you are wearing shoes and socks, can you take the shoes and socks off?
Carol:
Yes, sir.
Arvinder Walia:
Okay. Then, now get up and walk over to that corner please, and face the camera. That’s good. That’s good, just be comfortable.
Now, stretch your arms, if you can see me, all the way out. Okay, that’s good. You can rest.
Now I’d like to have a side view, so if you can face the wall, give enough space there, yeah just for right now, just be comfortable.
Now do the same thing with stretching your arms far out. Okay. We’re good. You can be comfortable back. Come back, have a seat, thank you again.
Okay. Uh, oh, I’ll have to ask you to get up, because I forgot to ask you to walk a little bit. So, go back in that corner. Walk towards the camera, turn around, go back towards the wall. Okay, perfect. You can come back and have a seat.
Carol:
OK, thank you.
Arvinder Walia:
Thank you.
Okay, so, the question is, I noticed some gait issues there. Are you having any problems with balance?
Carol:
I’m not having balance problems. My hips are hurting, because of osteoarthritis.
Arvinder Walia:
Okay, okay. Are you having any stiffness in any muscles, uh, throughout the body—muscle stiffness?
Carol:
No muscle stiffness.
Arvinder Walia:
Okay, okay.
All right, then. That’s pretty much all the questions that I had, uh, for you. That should be the end of our exam. Thank you again, Carol.
Carol:
Thank you, Dr. Walia.
Watch: Short Virtual Assessment
A virtual assessment without full view of the patient’s body.
For more videos, visit the YouTube page.
Arvinder Walia:
Good morning, Angie.
Angie:
Good morning, Dr. Walia.
Arvinder Walia:
How are you doing?
Angie:
I’m doing well, thank you.
Arvinder Walia:
Good, good. So, everything since our last visit, you feel has been going well? Your medicines are working okay?
Angie:
Yes, very good. No ups or downs and feeling very well.
Arvinder Walia:
Good. Good.
Uh, Angie, uh, one of the medicines that you take, uh, we have to watch out for certain side effects of that, uh, medicine.
So, what we have to do is periodically do a certain exam, uh, at, some regular intervals. So, I am going to be running you through that.
Um, it is going to be brief questions. Um okay, with that being said, here is my first question for you:
Have you or your family members noticed any abnormal movements either in your face, your arms, hands, or legs?
Angie:
No, not recently.
Arvinder Walia:
Okay. Okay. Well, let’s move further then.
Now before I start, um, quick question, anything in your mouth right now like a chewing gum or anything you have in your mouth?
Angie:
No.
Arvinder Walia:
Okay. Any dentures, uh, you wear…
Angie:
I have permanent implants.
Arvinder Walia:
And right now, they are in good shape, right?
Angie:
Yes.
Arvinder Walia:
Okay, all right. So, for the purposes of this exam, I’ll ask you if you can take your, uh, glasses off…
Now rest comfortably in the chair. Just make sure you don’t use the armrest for your arm, just let your arms rest on your legs.
Just be comfortable and I’m just going to be observing you for a few minutes here.
Okay. So, now what I want you to do is tap your right finger and thumb as I show you…
Start tapping it fast, keep doing it.
Move to the left, keep doing it. Okay, that’s good, you can rest back.
Now open your mouth wide open, that’s good. You can close it now. I’m going to ask you to open it again one more time, the same way. Okay, you can close it now.
So, I’m going to ask you to open your mouth again, but this go-around I’m going to ask you to stick your tongue out.
Good. You can close it. Open it one more time and stick your tongue out. Okay, you can close it.
We’re going back to the finger tap like we were doing, fast, now open your mouth as you are tapping it, yeah… stick your tongue out, keep tapping it, stick your tongue out. Okay, that’s good. You can rest.
Now, have you noticed any abnormal movement in your hands or in your arms?
Angie:
No, not lately.
Arvinder Walia:
Okay. Anything you’ve noticed like any abnormal movements in either your shoulders or your hips?
Angie:
No.
Arvinder Walia:
Any sort of strange abnormal movements in your legs, or your feet, or toes?
Angie:
No.
Arvinder Walia:
Okay, okay. Have you noticed any problems, uh, with your balance or gait?
Angie:
Yes, I do have some problems with my balance, um, I seem to sort of stumble to the right and then I have, um, trouble keeping my balance, like, on a bicycle and in restricted ways like that, I have a hard time keeping my balance.
Arvinder Walia:
Okay. Any stiffness in any part of the body or muscles that you’ve noticed?
Angie:
Yes, in my shoulders.
Arvinder Walia:
Tell me a little more about it.
Angie:
It seems to wax and wane. Um, I will have stiffness for several days at a time, and then it will go away, and then it will come back, and I will have it again for several days and then it will go away.
Arvinder Walia:
Is there any relationship in terms of timing? Uh, in other words, like stiffness comes, uh, comes in an hour or two after you take any of your medicines?
Angie:
No, not at all.
Arvinder Walia:
Okay. Okay. What about if there is any slowness in movements that develop, or sort of a fatigue, and slowness if you repeatedly continue to use a certain, uh, certain group of muscles?
Angie:
Yes, again, it is on my right side, and it’s in my arm a lot, and it just gets to be, where I know I’m not 100%.
Arvinder Walia:
Okay. Well, well, thank you Angie, uh, sort of, uh, ends our exam.
For more videos, visit the YouTube page.
Differentiating between TD and drug-induced parkinsonism (DIP) matters
The wrong treatment could lead to a worsening of symptoms20-22
Antipsychotic
discontinuation
Anticholinergics
VMAT2 inhibitors
TD
Not likely to improve TD
May worsen TD
Indicated for TD
Antipsychotic discontinuation
Anticholinergics
VMAT2 inhibitors
DIP
May improve DIP
Indicated for DIP
May worsen DIP
| TD | DIP | |
|---|---|---|
|
Nature of movements |

Movements are irregular, unpredictable, jerky, and twitchy20,21 |
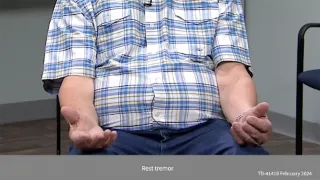
Movements are rhythmic20,21 |
|
Degree of movements |

Movements are excessive and continuous10 |

Overall paucity of movements23 |
|
Degree of muscle tone |
Normal muscle tone9 |
Stiffness and rigidity when joints are flexed9 |
|
Mechanism |
Dopamine blockade upregulates dopamine receptors and increases the potential for erratic dopamine signaling20 |
Dopamine blockade reduces dopamine signaling20 |
|
Timing of onset |
Delayed—usually occurs months or years following administration of antipsychotic therapy20 |
Acute—usually occurs within days or weeks following administration of antipsychotic therapy or increase in dose20 |
TD and DIP have different symptom onsets and movement types
TD
Delayed onset of symptoms20,21
The onset of symptoms occurs after using an antipsychotic for at least a few months to years.
- Elderly persons may develop TD symptoms in a shorter period of time
- In some patients, movements may appear after discontinuation or reduction in dosage
- If symptoms persist for longer than 4 to 8 weeks, it is considered TD
DIP
Early onset of symptoms20,21
The onset of symptoms occurs within a few weeks of any of the following:
- Starting a medication (eg, an antipsychotic)
- Increasing the dosage of a medication (eg, an antipsychotic)
- Reducing the dosage of a medication used to treat drug-induced movement disorders (eg, an anticholinergic)
TD
TD movements may include20
- Involuntary athetoid or choreiform movements in the face, jaw, trunk, or extremities
DIP
DIP movements may include21
- Parkinsonian tremor (rhythmic and faster than TD movements)
- Muscular rigidity
- Akinesia
- Bradykinesia
Listen: Experts address benztropine use in TD
Watch experts discuss TD and DIP differential diagnosis and the importance of appropriate treatment
Unraveling the Neurobiology of TD and DIP
Andrew Cutler:
Hello, I’m Dr Andrew Cutler, Chief Medical Officer at the Neuroscience Education Institute in Carlsbad, California, and a Clinical Associate Professor of Psychiatry at SUNY Upstate Medical University in Syracuse, New York.
Today, on behalf of Teva Pharmaceuticals, I’ll speak about the importance of differentiating tardive dyskinesia or TD, and drug-induced parkinsonism or DIP. We’ll take a look at how these disorders affect dopamine signaling to better understand why anticholinergics should not be used in patients with TD.
TD and DIP are common movement disorders caused by exposure to antipsychotic drugs.
It is unfortunate that the term extrapyramidal symptoms, or EPS, has historically been used to describe any drug-induced movement disorder, because it is a nonspecific term.
TD and DIP are both included under the umbrella of EPS and are often treated indiscriminately with anticholinergic medications. However, this concept is outdated. Today, we know these disorders are distinct and the use of anticholinergics for all drug-induced movements is inappropriate.
TD and DIP have different underlying causes, opposite clinical presentations, and very different treatments. In fact, treatment for 1 movement disorder may worsen the other.
Treatment guidelines from the American Psychiatric Association or APA and the DSM-5-TR provide recommendations for TD and DIP.
The APA guidelines for the treatment of schizophrenia recommend treating TD with a vesicular monoamine transporter 2, or VMAT2, inhibitor if symptoms have an impact on the patient, regardless of severity.
The DSM-5 cautions that symptoms of TD tend to be worsened by anticholinergic medications, such as benztropine.
Although anticholinergics are indicated for the treatment of DIP, the APA cautions that these agents can result in multiple difficulties for patients, including impaired cognition, diminished quality of life, and significant health complications.
The APA advises that it is important to keep in mind the total anticholinergic burden when selecting a medication for DIP. For this reason, antiparkinsonian medications are not typically administered on a prophylactic basis.
If an anticholinergic is used, it is important to adjust the medication to the lowest dose that can treat the symptoms, in order to minimize side effects. In addition, they should be used for the shortest time necessary.
Despite warnings about anticholinergic use, about 40% of psychiatry providers indicated they would initiate benztropine to treat, as well as to prevent, TD. Clearly the distinction between TD and DIP is misunderstood among the greater healthcare community.
The causes of TD and DIP can help explain their treatment. I hope that after watching this video you will have a better understanding of their biologic mechanisms, clinical symptoms, and appropriate treatments.
Patients with DIP display slowness of movement, or bradykinesia. I’ll demonstrate how this slowness, or as we see here, frozen appearance correlates with decreased dopamine.
In contrast, patients with TD have an excess of abnormal, irregular movements.
I’ll start with a refresher on the neurobiology of normal movement and then explain how signaling is altered in patients with DIP versus TD.
We will also review how anticholinergics resolve symptoms related to the mechanisms of DIP versus TD.
Movement is controlled by dopamine signaling in the basal ganglia. We’ll be discussing a presynaptic neuron that receives the signal to initiate movement and a postsynaptic neuron that transmits the signal to produce movement.
Here we can see that VMAT2 has transported dopamine into vesicles,
…where it waits for the signal to be released.
When the signal is received by the presynaptic neuron to initiate movement, the vesicles fuse with the presynaptic membrane and release dopamine into the synapse.
Dopamine binds to postsynaptic receptors…
and a signal is transmitted to initiate movement.
In DIP, antipsychotic drugs transiently block postsynaptic dopamine receptors.
When dopamine is now released, the antipsychotic drug is bound to dopamine receptors, and the blockade results in reduced dopamine signaling in the post synaptic neuron.
Reduced dopamine signaling results in bradykinesia, or slowness of movement.
Since the mechanism of DIP occurs when antipsychotics are first started, it makes sense that the clinical signs of DIP occur early, within a few weeks to months of starting or increasing the dosage of an antipsychotic.
The exact pathophysiology of TD is not fully known. However, it is thought that chronic blockade of dopamine receptors by antipsychotics results in the upregulation, or a hypersensitivity, of dopamine receptors.
This upregulation results in increased dopamine signaling, which manifests as the excessive movements that are characteristic of TD, which is a possible explanation for why DIP is a risk factor for TD.
In TD, a chronic blockade of dopamine receptors by antipsychotics results in an upregulation, or hypersensitivity, of dopamine receptors. So, TD develops after using an antipsychotic for at least a few months, typically years.
Some elderly or high-risk patients may develop symptoms in a shorter period.
Discontinuing the antipsychotic may improve or resolve the symptoms associated with DIP.
In contrast, reducing the dose or discontinuing an antipsychotic may fail to improve the symptoms of TD or might even induce withdrawal dyskinesia.
Anticholinergic agents like benztropine act to promote dopamine signaling. This can help alleviate the bradykinesia associated with DIP, but it is the exact opposite of what we want to do with TD.
If we add more dopamine to the postsynaptic neuron in TD, the already upregulated receptors may transmit an even greater number of signals. This could result in an even further increase in abnormal movements.
As mentioned previously, guidelines recommend VMAT2 inhibitors for adults with TD.
It is believed that VMAT2 inhibitors reduce the amount of dopamine packaged into presynaptic vesicles, which may reduce the amount of dopamine released at the synapse and reduce TD movements.
If we use a VMAT2 inhibitor in a patient with DIP, it could worsen symptoms, as reduced levels of dopamine being released into the synapse can reduce the already decreased dopamine signaling and further slow the patient’s movement.
Recognizing the different neurobiology and clinical presentation between TD and DIP can help clinicians choose appropriate treatment.
I hope this video helped you understand these differences and will encourage you to apply this knowledge to help your patients
Differentiating the Clinical Presentations of TD and DIP
Rajeev Kumar:
Hello. I’m Dr Rajeev Kumar, the medical director of the Rocky Mountain Movement Disorders Center and the Huntington’s Disease Society of America, Center of Excellence in Englewood, CO. I’ve been working with patients with tardive dyskinesia, or TD, for more than 20 years.
Today, on behalf of Teva, I'll be speaking about clinical symptoms and treatment of TD and its differentiation from drug-induced parkinsonism, or DIP. Movement disorders in patients taking antipsychotics are common and up to one-third of them will develop either TD, DIP, or both.
I’ll be focusing on how these 2 disorders may be misdiagnosed, and how treating a patient with TD for DIP could have adverse consequences for the patient. I’ll also speak about how the opposite is true as well.
Tardive dyskinesia and drug-induced parkinsonism are both very common movement disorders, and both are caused by dopamine receptor blocking medications, like antipsychotics.
However, they present differently, differ in their pathophysiology with respect to dopaminergic signaling, and have different treatments. In fact, treatment for one disorder may worsen the other.
The term EPS, or extrapyramidal symptoms, has been used in psychiatry for many years, and this term has been propagated by the labeling of medications by the FDA.
Historically, EPS has been used to describe all drug-induced movement disorders experienced by patients taking antipsychotic medications. As a result, there’s been the indiscriminate treatment of both disorders with anticholinergic medications.
We now know that the term EPS is outdated. It’s very important to differentiate between the disorders because they merit different treatments.
The use of antipsychotics has markedly increased over the past 2 decades due to expanded indications for use in mood disorders, such as depression and bipolar disorder.
The data suggests that in 2022, 8.7 million Americans received prescriptions for an antipsychotic and, as a result, we estimate there are at least 785,000 individuals who have TD in the United States.
Now that's a shocking number, and even more surprisingly, at least 85% of patients have been misdiagnosed or remain undiagnosed.
Many of these individuals, who have been misdiagnosed, have been labeled as having EPS, and many of them, in fact, have DIP.
So, this means that about 15% of patients have been diagnosed and only 6% of individuals have been treated with a VMAT2 inhibitor.
If we look at US healthcare claims data of those individuals who have been diagnosed with TD, about 36% of individuals are receiving benztropine with about three-quarters of these individuals having received benztropine for more than 3 months, and about a-third having been treated for greater than a year.
And if we look at online surveys of psychiatry providers, about 40% of individuals indicate that they would actually initiate benztropine to treat TD, which is an unfortunate failure of our ability to educate these providers.
So, for patients suffering from TD, the lack of an accurate diagnosis can lead to mistrust and frustration. And of course, for patients that have been misdiagnosed, the wrong therapy can result in a worsening of their symptoms.
As a result, patients can fail to seek care because of their frustration and may not follow instructions correctly.
Let's talk a little bit about the underlying mechanisms of disease for both TD and DIP, and then we'll review some videos that could help one make appropriate diagnostic and treatment decisions when seeing patients with drug-induced movement disorders.
First, let's talk about the time course of TD and DIP. DIP occurs due to acute blockade of predominately dopamine D2 receptors.
Now, because of this blockade, we get a reduction in postsynaptic dopamine signaling.
Tremor, bradykinesia, rigidity, and impairment of gait may become apparent within the first few weeks or first few months of starting or increasing the dose of the antipsychotic.
In tardive dyskinesia, the term “tardive” means delayed. Chronic blockade of dopamine receptors by antipsychotics results in delayed upregulation, or super sensitivity, of these postsynaptic dopamine receptors. So, TD usually develops after months or years of exposure to antipsychotic medications.
Let's review the clinical features of DIP and TD and see how they're differentiated when we examine patients.
The degree of movements is one of the first things I look for.
This patient with DIP has a masked face with reduced facial expression and reduced blink rate, which is different from the typical normal level or increased level of facial movement we see in TD.
This patient also has mildly flexed posture, reduced arm swing, reduced stride length, reduced speed, and multi-step turns, all of which are characteristic of parkinsonism.
The nature of movements in DIP and TD are also different. When patients with DIP have abnormal movements, it is rhythmic.
This elderly gentleman has typical 4 to 5 Hertz resting tremor in the left hand, and it is not uncommon for the tremor to improve during sustained action and posture, but it may persist.
You can see this with the postural tremor in this gentleman's right hand.
And in fact, if we allow the jaw to hang down, he has synchronous tremor of his jaw.
TD is characterized by excessive movement or hyperkinesia.
This patient has continuous movements of the neck, jaw, and face. We can see she has quivering movements of the lips. In addition, she has excessive right shoulder elevation and right head tilt.
This patient has undulating movements of the lips, occasional tongue protrusion, and excessive blinking.
This younger woman has excessive lip pursing, platysma contraction, frontalis activation, and eyebrow elevation.
This patient has tongue movements that are unique. They go back and forth and then to one side of his mouth. He has very severe back and forth movements of his tongue, which are unpredictable, and, in fact, you can see that he has involuntary jaw closing and briefly bites his tongue.
This patient’s TD movements are irregular, jerky, and unpredictable. This patient has severe generalized choreiform movements, with piano playing movements in the fingers and the toes, body rocking, and facial dyskinesias with grimacing, and we can see the protrusion of his tongue within the mouth.
Let's look at the upper extremity muscle tone, which is a component of the AIMS examination.
First, we examine rigidity at the level of the wrist by isolating the wrist and passively moving it. Then we examine the tone at the level of the elbow.
Now, if you do not detect increased tone at rest, have the patient open and close the other hand, while we examine the tone again at the level of the wrist and the elbow.
We should do this for each side of the body independently. As you can see, I can easily move this TD patient’s arm at the level of the wrist and the elbow without resistance, both with and without activation of the contralateral hand.
Performing the same maneuvers on this man with parkinsonism, you can see that I don't have difficulty moving his wrist, but there is a resistance that occurs when I attempt to flex and extend his elbow.
Now, this is something you predominantly feel, but here you can almost see that I'm having some difficulty extending his elbow.
To make an accurate diagnosis of TD, it is essential that we are able to carefully assess and differentiate TD from DIP.
Because if one makes a misdiagnosis and prescribes an anticholinergic to a patient with TD, you may exacerbate the hyperkinetic movements by increasing dopaminergic signaling.
On the other hand, if you prescribe a dopamine-depleting VMAT2 inhibitor to a patient with DIP, you further reduce the patients already low level of dopamine, and this can worsen the patients’ features of parkinsonism.
So, it's important to differentiate TD and DIP by careful observation and examination of the patient. With the correct diagnosis, we can render the correct treatment.
Thank you for watching, and I hope what I’ve shared today will help you in the assessment of your patients with movement disorders who are taking antipsychotic medications.
Deciphering the Diagnostic Landscape: TD and DIP
Craig Chepke:
Hi, I’m Dr. Craig Chepke, adjunct professor of psychiatry for Atrium Health and medical director of Excel Psychiatric Associates in Huntersville, North Carolina.
Today, on behalf of Teva, I’ll talk about the importance of differentiating tardive dyskinesia, or TD, and drug-induced parkinsonism, or DIP. The American Psychiatric Association, or APA, and the DSM-5-TR, provide distinct treatment recommendations for TD and DIP, but there is a disconnect between the guidelines and how patients are being managed in the real world. Data indicate that anticholinergics are being used to treat TD, but this is not an effective treatment strategy and can have a number of detrimental consequences for patients.
Approximately one-third of patients taking antipsychotic drugs, or APDs, will experience TD, DIP, or both.
In the past, TD and DIP were both described by the term “EPS,” or extrapyramidal symptoms, and as such, patients with both disorders were managed with anticholinergics.
However, the concept of EPS is outdated. We now understand that TD and DIP have different underlying causes, opposite clinical presentations and, therefore, very different treatments.
Unfortunately, the concept of EPS is so well entrenched in psychiatry, and many providers still don’t recognize that TD is distinct from DIP.
It’s critical to differentiate patients with TD from patients with DIP, because treatment for one disorder may exacerbate the other disorder.
The APA Practice Guideline for the Treatment of Schizophrenia and the DSM-5-TR guidelines provide guidance for the treatment of each disorder, as well as several precautions that should be made when considering anticholinergic medications.
VMAT2 inhibitors, which are approved for TD, are recommended if symptoms have an impact on the patient.
In contrast, symptoms of TD tend to be worsened by anticholinergic medications, such as benztropine.
Anticholinergics are indicated for the treatment of DIP; however, their use has been linked to impaired quality of life, impaired cognition, and significant health complications.
Guidelines recommend considering the anticholinergic burden before prescribing a medication in this class. Many medications have anticholinergic properties, and for this reason, anticholinergics are not typically administered prophylactically when starting an antipsychotic.
If an anticholinergic is used, it should be adjusted to the lowest dose possible for the shortest time necessary.
Despite the recommendations regarding the use of anticholinergics, a survey of providers indicated that fewer than 40% are familiar with the 2020 APA Practice Guideline for the Treatment of Schizophrenia.
In the United States, TD affects approximately 785,000 patients.
However, only about 15% of these patients have received a formal diagnosis…
and less than 6% have been treated with a VMAT2 inhibitor.
So, this leaves as many as 85% of patients with TD either being missed, or possibly, misdiagnosed with DIP.
In addition, approximately 40% of psychiatric providers stated they use benztropine to prevent or treat TD.
In a separate analysis of patients who were diagnosed with TD, 36% were receiving benztropine. Additional analyses from the same data set indicated that, of those patients with TD who were taking benztropine, about 75% of them remained on treatment for over 3 months, and 35% were treated for over a year.
Training and education to reduce use of benztropine in TD is needed because clinical symptoms can be tied to the relative changes in dopamine signaling that can occur. I’ll be reviewing the mechanisms underlying TD and DIP to explain why treating patients for TD with anticholinergics can worsen symptoms of TD. Recognizing the unique symptoms and neurobiology of both disorders may help clinicians align with the treatment guidelines.
The time that it takes for symptoms of these disorders to present after use of antipsychotic drugs differs along with their clinical presentations.
Parkinsonian symptoms generally begin within a few weeks to a few months of starting or increasing the dosage of an antipsychotic drug.
DIP occurs due to an acute blockade of dopamine receptors, which leads to a reduction in postsynaptic dopamine signaling.
On the other hand, in TD, the chronic blockade of dopamine D2 receptors by antipsychotics result in an upregulation and hypersensitivity of dopamine receptors.
As such, TD develops after using an antipsychotic drug for at least a few months, but more typically over a few years. By DSM-5-TR criteria, a diagnosis of TD requires at least 3 months of exposure to antipsychotics for adults, but as little as 1 month for elderly patients.
The symptoms of DIP are correlated with a decrease in dopamine signaling, whereas the symptoms of TD are correlated with a relative increase in dopamine signaling. We generally see reduced movement in patients with DIP vs excessive movement in TD.
DIP is characterized by a paucity or slowness of movement. The person in this video demonstrates a masked face consistent with DIP; she appears to be staring and rarely blinks.
In contrast, a person with TD has movements that are excessive and often continuous.
This patient has an increased rate of blinking. Instead of a frozen look, she is puckering her lips repeatedly.
The nature of movements, whether hyperkinetic or hypokinetic, is one of the most important features to help me differentiate TD from DIP.
While DIP is generally associated with a paucity of movement, patients with DIP may sometimes have a tremor; however, the nature of that tremor is different from the movements of TD.
The patient has a parkinsonian tremor in his left hand, which occurs in a predictable and rhythmic fashion.
In contrast, the movements of TD are unpredictable, irregular, and sometimes even jerky. This patient has frequent involuntary movements, especially notable in his fingers and toes.
Evaluating the patient’s muscle tone is also helpful in differentiating TD from DIP.
DIP may be accompanied by rigidity that can be felt and confirmed in a physical exam.
In contrast, patients with TD have normal muscle tone.
Discontinuing the APD may improve or resolve the symptoms associated with DIP.
But if left untreated, TD is typically persistent and irreversible. APD reduction or withdrawal may fail to improve the symptoms of TD or might even induce withdrawal dyskinesia.
Based on the differences between TD and DIP, we must consider which treatment approach can best address this specific condition.
Anticholinergics are indicated for the treatment of DIP. However, the guidelines and the label for benztropine, which is a commonly used anticholinergic, warn that these agents do not alleviate the symptoms of TD and in some cases, may even aggravate them.
VMAT2 inhibitors are recommended for adults with TD but they do have the potential to worsen DIP.
Contrary to recommendations made in treatment guidelines, anticholinergics are being overprescribed in patients with TD, and only a fraction of patients with TD are being treated with a VMAT2 inhibitor.
The APA and DSM-5-TR guidelines reinforce that TD and DIP are 2 very different disorders with different proposed pathophysiologies. As such, it’s critical that we make the right diagnosis so we can select the right treatment approaches for our patients.
I hope this video has provided a helpful overview of these two disorders and will be useful in the clinical management of your patients.
Visit the YouTube page to view Demystifying EPS, a 3-part series on the importance of differential diagnosis.
Assessing the impact of TD is imperative for optimal management
- The overall impact of TD on key functional domains—social, physical, vocational, and psychological/psychiatric—should be assessed at every patient visit24
- The degree of impact helps determine the urgency with which symptoms should be addressed24
VMAT2 inhibitors—like AUSTEDO XR—are recommended by the APA as a first-line treatment option for patients impacted by TD1

Download the IMPACT-TD Scale, which was developed by a consensus panel to evaluate how TD affects patients’ lives
No clinical trials have been conducted to demonstrate that treating TD affects the outcomes presented on this page.
EPS, extrapyramidal symptoms; VMAT2, vesicular monoamine transporter 2.
REFERENCES: 1. American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. American Psychiatric Association; 2021. 2. Gharabawi GM, Bossie CA, Lasser RA, Turkoz I, Rodriguez S, Chouinard G. Abnormal Involuntary Movement Scale (AIMS) and Extrapyramidal Symptom Rating Scale (ESRS): cross-scale comparison in assessing tardive dyskinesia. Schizophr Res. 2005;77(2-3):119-128. 3. Guy W. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare; 1976. 4. Abnormal Involuntary Movement Scale (AIMS). Oregon Health & Sciences University website. Accessed March 26, 2025. https://www.ohsu.edu/sites/default/files/2019-10/%28AIMS%29%20Abnormal%20Involuntary%20Movement%20Scale.pdf 5. AUSTEDO XR® (deutetrabenazine) extended-release tablets and AUSTEDO® current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc. 6. Caroff SN, Citrome L, Meyer J, et al. A modified Delphi consensus study of the screening, diagnosis, and treatment of tardive dyskinesia. J Clin Psychiatry. 2020;81(2):19cs12983. 7. Abdo WF, van de Warrenburg BPC, Burn DJ, Quinn NP, Bloem BR. The clinical approach to movement disorders. Nat Rev Neurol. 2010;6(1):29-37. 8. Munetz MR, Benjamin S. How to examine patients using the Abnormal Involuntary Movement Scale. Hosp Community Psychiatry. 1988;39(11):1172-1177. 9. Data on file. Parsippany, NJ: Teva Neuroscience, Inc. 10. Caroff SN. Overcoming barriers to effective management of tardive dyskinesia. Neuropsychiatr Dis Treat. 2019;15:785-794. 11. Hoberg A. ‘Activation maneuvers’ can help identify mild to moderate TD. Psych Congress Network. June 8, 2023. Accessed November 15, 2024. https://www.hmpgloballearningnetwork.com/site/pcn/videos/activation-maneuvers-help-identifying-mild-moderate-td 12. Jain R. Can the AIMS exam be conducted via telepsychiatry? Psych Congress Network. December 9, 2019. Accessed November 15, 2024. https://www.hmpgloballearningnetwork.com/site/pcn/article/can-aims-exam-be-conducted-telepsychiatry 13. Amarendran V, George A, Gersappe V, Krishnaswamy S, Warren C. The reliability of telepsychiatry for a neuropsychiatric assessment. Telemed J E Health. 2011;17(3):223-225. 14. McEvoy JP. The assessment of tardive dyskinesia via telepsychiatry. Clinical Care Options. May 4, 2021. Accessed November 15, 2024. https://clinicaloptions.com/CE-CME/assessing-td-via-telepsychiatry/100003953-100008679 15. Dorsey R, Fahn S, Poplar T. Can we make a new diagnosis and treat Parkinson’s disease by telemedicine? International Parkinson and Movement Disorder Society. March, 2021. Accessed November 15, 2024. https://www.movementdisorders.org/MDS/Scientific-Issues-Committee-Blog/cwmandtpdbt.htm 16. Drerup B, Espenschied J, Wiedemer J, Hamilton L. Reduced no-show rates and sustained patient satisfaction of telehealth during the COVID-19 pandemic. Telemed J E Health. 2021;27(12):1409-1415. 17. Citrome L. Treating TD in the COVID-19 era: 5 steps to success. Psychiatry and Behavioral Health Learning Network. June 8, 2020. Accessed November 15, 2024. https://www.hmpgloballearningnetwork.com/site/pcn/multimedia/treating-td-covid-19-era-5-steps-success 18. Shore J, Vo A, Yellowlees P, et al. Antipsychotic-induced movement disorder: screening via telemental health. Telemed J E Health. 2015;21(12):1027-1029. 19. Jackson R. Dr Richard Jackson on assessing and monitoring TD through telepsychiatry. Psychiatry and Behavioral Health Learning Network. June 15, 2020. Accessed November 15, 2024. https://www.hmpgloballearningnetwork.com/site/pcn/multimedia/dr-richard-jackson-assessing-and-monitoring-td-through-telepsychiatry 20. Ward KM, Citrome L. Antipsychotic-related movement disorders: drug-induced parkinsonism vs tardive dyskinesia—key differences in pathophysiology and clinical management. Neurol Ther. 2018;7(2):233-248. 21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. 22. Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013;3:tre-03-161-4138-1. 23. Patel T, Chang F. Practice recommendations for Parkinson’s disease: assessment and management by community pharmacists. Can Pharm J (Ott). 2015;148(3):142-149. 24. Jackson R, Brams MN, Citrome L, et al. Assessment of the impact of tardive dyskinesia in clinical practice: consensus panel recommendations. Neuropsychiatr Dis Treat. 2021;17:1589-1597.


